
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
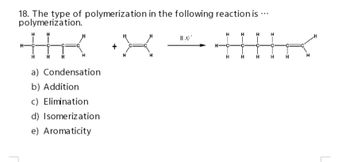
Transcribed Image Text:18. The type of polymerization in the following reaction is ...
polymerization.
H
a) Condensation
b) Addition
c) Elimination
d) Isomerization
e) Aromaticity
11.0'
H
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Use the web to research the amount of PVC polymer produced annually in the United States. What are the three most common uses of this polymer?arrow_forward2. In your own words, explain the difference between step-reaction polymerization and chain-reaction poly- merization. What is the advantage of this terminology over the more traditional addition and condensa- tion polymerization?arrow_forwardWhich polymer below is the same as this? H 'N' in A) B) C) D) N’ H un up ZIarrow_forward
- Q2. A) Determine the monomer unit of the following polymer Monomer unit = B) Determine the structure of polymer that will be synthesized from the given monomer unit Q2. A) Determine the monomer unit of the following polymer CH3 CH3 CH3 (CH2-C-CH₂-C-CH₂-07 CO2CH3 CO2CH3 CO2CH3 Monomer unit = B) Determine the structure of polymer that will be synthesized from the given monomer unit Initiator heatarrow_forwardThis polymer is composed of 2 monomer units: an acid chloride and an amine. In the box below, draw the structure of both monomers. (8-10 C (CH₂)6-C CH₂ 어 H n • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu.arrow_forward10. Complete the following polymerization reactions. Name the polymer that forms. a) H b) H H H H H polymerize polymerize polymerize CH3 -CH-CH₂arrow_forward
- General polymerization processes. Describe stepwise polymerization and chain polymerization.arrow_forwardComplete the following condensation polymerization reactions by drawing the structural formula of the repeating unitof each polymer, as well as any other products.arrow_forward3. Another type of Nylon can be made from 1,4-butanediamine and sebacoyl chloride: | CI - C(CH2);C –- CI H2N(CH2)4NH2 and a. Draw the structure of the polymer formed. Draw parentheses around the repeating unit. b. Is this an addition polymerization or a condensation polymerization reaction? (Circle your choice) c. What molecule will be eliminated in this reaction? d. What name (with numbers) would you give to this polymer? In the repeating unit, circle and label the amide bond. е.arrow_forward
- Please long and clear explanations.arrow_forwardFor an alkene to polymerize easily via cationic polymerization... A) Atoms adjacent to the carbocation should have 6- B) Atoms adjacent to the carbocation should have &+ C) Atoms adjacent to the carboanion should have 8- D) Atoms adjacent to the carboanion should have &+arrow_forward6. Dacron is the brand name for the polymer that is made from ethane-1,2-diol and benzene-1,4-dicarboxylic acid. (a) When the two monomers combine, what type of reaction do they undergo and what molecule is eliminated? [1] (b) What is the name for the linkages that join the monomers together? [1] (c) Draw the monomers and the polymer of the reaction to create Dacron. [3]arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning