
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
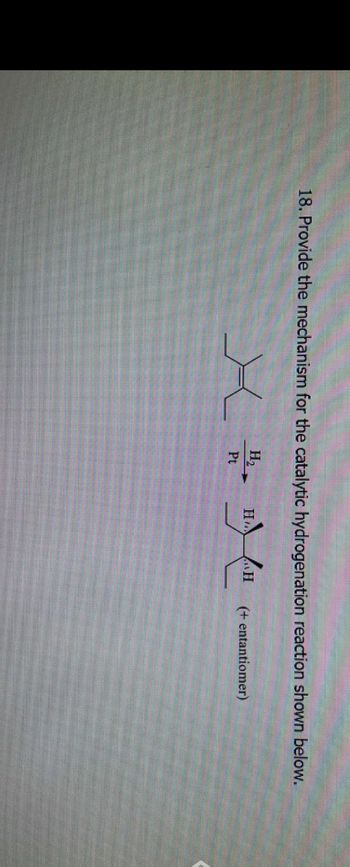
Transcribed Image Text:18. Provide the mechanism for the catalytic hydrogenation reaction shown below.
H₂
Pt
X
H₂
H
(+ entantiomer)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the followsing reactionse a. CH20H + CH3 CHz_ott Ho Cit2OH CHO otH +Cu+ CH20H CH3 + Cltz CH -OH HF OHarrow_forwardComplete the given reactions by drawing the structures of the major organic products: Please draw the completed reaction OH HO HOCN H₂N H₂O H3PO4 OH HO HO. (excess) H3O+ H2SO4 H*arrow_forwardWhat reagent)(s) would accomplish the conversion shown below? ? cat. OsO4, H202 Ozone, then (CH3)3S RCO3H H2O2, NaOHarrow_forward
- 1. Cl2, H20 2. NaOH Another mechanism for the formation of epoxides is through the formation of a chlorohydrin. Alkenes react with chlorine in the presence of H,0 to give a chlorohydrin via a cyclic chloronium ion intermediate. When the chlorohydrin is treated with strong base, HCl is eliminated and the epoxide is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructionsarrow_forwardto 1. conc. HBr H20 Br OH O Na 2. Na NH2 NH3 d = El Elimination f = SN1 Nucleophilic substitution a = Proton transfer b = Lewis acid/base e = E2 Elimination g = SN2 Nucleophilic substitution c = Electrophilic addition The rections above involve synthesis or reactions of alcohols and ethers. Identify the mechanism by which they proceed from among the mechanisms listed. Use the letters a - g for your answers. 1. 2. Submit Answer Retry Entire Group 8 more group attempts remainingarrow_forwardClassify each of the following species as a nucleophile or an electrophile CH3 CH3-CECH CH3- -CH3 O:c EN: CH3-0-CH3 | I II III IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY