Question

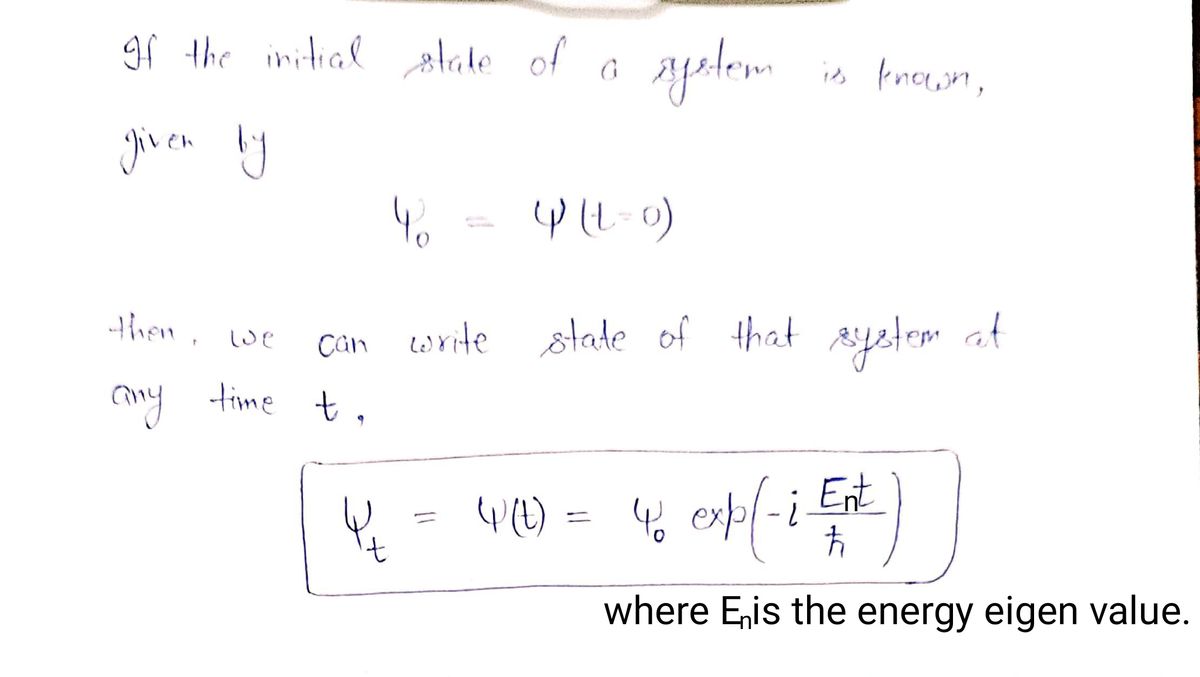
Transcribed Image Text:18. If the initial state of a system is known, given by Y(t = 0)
Y(t)=
where
and
Wn =
Cn=
HPn=
=
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Determine the signs of (d) Q, (e) W, and (f) ΔU associated with process BC. D) q, posative, negative, or zero E) w, posative, negative, or zero F) Δu, posative, negative, or zeroarrow_forwardWater vapor at a pressure 0f 2X10 N/m2 0nd a dempirature of as0°c is 0.32 x106 N/m2.following expanded to a yoessune of ec.For the low P.V1.25 this expanston determine: a. Pinal vapor condnion b. Syecipie haat transpan c. Changis in tntroyy SIDUarrow_forwardA modern-day zeppelin holds 7,620 m3 of helium. Compute its maximum payload at sea level. (Assume the helium and air to be at 0°C and 1 atm.) Narrow_forward
- Thank you! a) what is the kinetic energy per unit volume in an ideal gas at P=1.20 atm? b) what is the kinetic energy per unit volume in an ideal gas at P=287.0 atm?arrow_forwardThe heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) Figure p (kPa) 600 400 200 0 0 0.025 0.050 V (m³) 1 of 1 Part A Determine T₁, T2, and T3. Enter your answers numerically separated by commas. Express your answer using two significant figures. T₁, T2, T3 = 600,1800,1200 K Submit Previous Answers Correct Part Barrow_forward2arrow_forward
arrow_back_ios
arrow_forward_ios