
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Show work with explanation needed. Don't give Ai generated solution
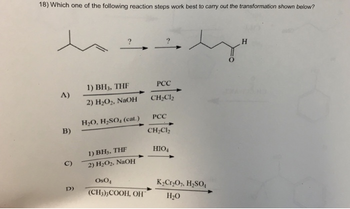
Transcribed Image Text:18) Which one of the following reaction steps work best to carry out the transformation shown below?
1) BH, THE
PCC
A)
2) H2O2, NaOH
CH2Cl2
H2O, H₂SO (cat.)
PCC
B)
B)
CH2Cl2
1) BH3, THF
HIO4
C)
2) H2O2, NaOH
D)
OsO
K2Cr2O7, H₂SO₁
(CH3)3COOH, OH
H₂O
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 18. Which one of the following reaction steps work best to carry out the transformation shown below? A) B) D) 1) BH₂, THF 2) H₂O₂, NaOH H₂O, H₂SO₂ (cat.) 1) BH₂, THE 2) H₂O₂, NaOH Os0₁ (CH3),COOH, OH PCC CH₂Cl₂ NaBH4 H₂O A) Cyclopentene oxide PCC CH₂Cl₂ HIO₂ K₂Cr₂O₂, H₂SO, H₂O 19. What is the product of the following reaction sequence? H₂SO4 B) C) cyclopentane 20. Which is not true for Thiols (RSH)2 A) More acidic than alcohol. B) lower boiling than alcohols C) Less water soluble than alcohols D) Less acidic than alcohols heat H B) cyclopentene D) 1,2-cyclopentanediolarrow_forwardFrom the following reaction are step 1 and 2 correct?, explain why or why not and draw the whole mechanismarrow_forwardWhich conditions will most efficiently affect the transformation shown? A) NaBH₁, CH₂OH B) PCC НО C) CrO3, H₂SO4 D) 1. CH₂MgCl, 2. H₂O+arrow_forward
- Rank the following molecules relative reactivity from slowest to fastest under SN1. Br A [Select] [Select] Br B Br [Select] [Select] C Br Hy D H Varrow_forwardDecide which of the following molecules these statements belong to. Each molecule is only used as answer once. Br A B Br C Br D This molecule has the highest reaction rate of [Choose ] all in an SN2 reaction. [Choose ] This molecule has the highest reaction rate in an D SN1 reaction. A This molecule can neither react by an SN1 nor an SN2 mechanism. E B This molecule can react by an SN1, E1 or E2 mechanism but will never react by an SN2 mechanism. C F This moelcule can react my an SN2 or E2 mechanism but will not react by an SN1 or E1 mechanism. [Choose] This molecule reacts rapidly by either an SN1 or [Choose ] an SN2 mechanism. له Br Br E F Brarrow_forward9. Complete Reactions. Provide only the major product for E2&SN2 reactions. E1/SN1 provide all possible products. NaOH Br provide mechanism CI Provide mechanism + CI NaOCH 3 KCNarrow_forward
- Rank the following molecules in order of increasing relative rate of SN1 reaction with methanol, CH3OH (slowest to fastest reacting). Br Br CH3-Br 1 X X X Br Br 2 5 4 3 C) 5<4<3<2<1 A) 1<2<4<3<5 D) 4 <2<3<5<1 B) 5 < 1<2<4<3 E) 1<2<5<3 <4arrow_forward2. Draw the structures and explain why CH3CH₂O and CH3CO₂ are good nucleophiles but CH3SO3, water, and alcohols (R-OH) are poor nucleophiles. Propose a 'cutoff' for the amount of negative charge needed to be a good nucleophile. CH3CH₂O CH3CO₂ CH3SO3 H₂O CH₂OHarrow_forwardUse the E2 mechanism to explain why when I is mixed with sodium ethoxide (NaOEt) in ethanol, the major product is III, but when II is mixed with sodium ethoxide in ethanol, the major product is IV. H3C CH3 H3C CH3 ..CI CI CH3 I CH3 || H3C. CH3 H3C CH3 E ||| CH3 CH3 IV SANarrow_forward
- Your task is to convert 2-bromobutane to 1-butene in highest yield. Which reagents would you use? O KOH/H2O O KOH/CH,OH O CH3CH2ONA/CH3CH2OH O CH3ONA/CH3OH O (CH3)3COK/(CH3)3COHarrow_forwardDRAW a stepwise reaction mechanism for this reaction. NO₂ -N3 C c 03, NaHCO3, CH2C12/MeOH. then Ac20, Et3N NO₂arrow_forwardHO. Provide a detailed, step-by-step mechanism for the reaction shown below. Br₂ Br HBrarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning