
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
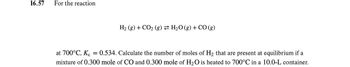
Transcribed Image Text:16.57
For the reaction
H₂ (g) + CO₂ (g) H₂O(g) + CO (g)
at 700°C, Kc = 0.534. Calculate the number of moles of H₂ that are present at equilibrium if a
mixture of 0.300 mole of CO and 0.300 mole of H₂O is heated to 700°C in a 10.0-L container.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The equilibrium constant, K, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H₂(g) + 1₂(g) ? An equilibrium mixture of the three gases in a 1.00 L flask at 698 K contains 0.330 M HI, 4.43×10-² M H₂ and 4.43×10-2 M 1₂. What will be the concentrations of the three gases once equilibrium has been reestablished, if 3.69x10-2 mol of H₂(g) is added to the flask? [HI] = [H₂] = [1₂] = ΣΣΣarrow_forward-2 The equilibrium constant K,, for the following reaction is 7.73×10atmat 350°C. What is the value of K. for the reaction at the same temperature? N2(g) +3H2(g)→ 2NH3(g)arrow_forwardEquilibrium constant, Kc, for the following reaction is 8.08×10-3 at 1080 K.2SO3(g) 2SO2(g) + O2(g)Calculate the concentration of SO3 in the equilibrium mixture?When a sufficiently large sample of SO3(g) is introduced into an evacuated vessel at 1080 K, the equilibrium concentration of O2(g) is found to be 9.70×10-2 M.arrow_forward
- A.) The equilibrium constant, K, for the following reaction is 2.90×10-2 at 1.15×103 K.2SO3(g) 2SO2(g) + O2(g)If an equilibrium mixture of the three gases in a 16.3 L container at 1.15×103 K contains 0.431 mol of SO3(g) and 0.309 mol of SO2, the equilibrium concentration of O2 is ______________________ M. ( fill in the blank ) B.) The equilibrium constant, K, for the following reaction is 6.50×10-3 at 298 K.2NOBr(g) 2NO(g) + Br2(g)If an equilibrium mixture of the three gases in a 19.7 L container at 298 K contains 0.433 mol of NOBr(g) and 0.500 mol of NO, the equilibrium concentration of Br2 is_______________________ M. ( fill in the blank)arrow_forwardA mixture of 1.374 g of H2 and 70.31 g of Br2 is heated in a 2.00 L vessel at 700 K. These substances react according to H2 (g) + Br2 (g) 2HBr (g). At equilibrium, the vessel is found to contain 0.566g of H2. Calculate equilibrium concentrations for H2, Br, and HBr.arrow_forwardSulfuryl chloride, SO2Cl2 , is a compound with very irritating vapors; it is used as a reagent in the synthesis of organic compounds. When heated to a sufficiently high temperature, it decomposes to SO2 and Cl2 . An 1.00-L flask containing 4.93 g of SO2Cl2 is heated to 375 °C. What is the concentration of SO2Cl2 in the system when equilibrium is achieved?arrow_forward
- At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, Kc. 3A(g) + 2B(g)↽−−⇀4C(g) ?c=2.13×10^25 If, at this temperature, 2.30mol of A and 3.60mol of B are placed in a 1.00 L container, what are the concentrations of A, B, and C at equilibrium? [A]= M [B]= M [C]= Marrow_forwardThe equilibrium constant K = 53.09 for H2(g) +12(g) 2 HI(g) has been determined at a certain temperature. If 1.00 mol each of H2 and 12 is placed in a 0.500-L flask at that temperature, what are the concentrations of H2, 12, and HI when equilibrium has been achieved? [H₂]= M [12]= M [H0] => Marrow_forwardPhosphorus pentachloride is formed when phosphorus trichloride and chlorine react according to the following reaction. PCI2(g) + Cl2(g) ⇌ PCI5(g) The equilibrium constant for the reaction is Kc = 43.7 at 130 °C. If 0.587 mo/ of phosphorus trichloride is added to 0.423 mol of chlorine in a 1.26-L reaction vessel and allowed to react at this temperature, what is the equilibrium concentration (in mol/L) of chlorine? Report your answer to THREE significant figures.arrow_forward
- The equilibrium constant, Kc , for the following reaction is 7.00×10-5 at 673 K.NH4I(s) NH3(g) + HI(g)If an equilibrium mixture of the three compounds in a 4.05 L container at 673 K contains 2.71 mol of NH4I(s) and 0.262 mol of NH3, the number of moles of HI present is moles. --- The equilibrium constant, Kc, for the following reaction is 6.30 at 723K.2NH3(g) N2(g) + 3H2(g)If an equilibrium mixture of the three gases in a 12.1 L container at 723K contains 0.250 mol of NH3(g) and 0.208 mol of N2, the equilibrium concentration of H2 is M.arrow_forwardConsider the reaction: 3H2(g)+N2(g)=2NH3(g) If this reaction is at equilibrium and the pressure is decreased, what will happen to the concentration of H2 and NH3?arrow_forwardThe following reaction is important in the production of sulfuric acid. 2SO2(g) + O2(g) 2SO3(g) At 627 °C, 0.0291 mol SO2, and 0.0148 mol O₂ are sealed in a 1.50 L reaction vessel. When equilibrium is reached at the temperature of 725 °C, the concentration of SO3 is determined to be 0.0175 M. What is the value of Ke for the reaction? Calculate the Kp for the reaction at 725 °C. (17 pts.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY