
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Answer number 16.8
Transcribed Image Text:16: Amino Acids, Proteins, and Enzymes
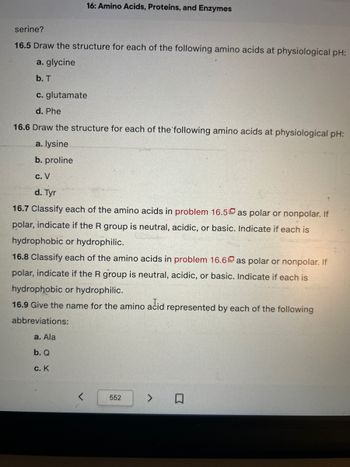
serine?
16.5 Draw the structure for each of the following amino acids at physiological pH:
a. glycine
b. T
c. glutamate
d. Phe
16.6 Draw the structure for each of the following amino acids at physiological pH:
a. lysine
b. proline
c. V
d. Tyr
16.7 Classify each of the amino acids in problem 16.5 as polar or nonpolar. If
polar, indicate if the R group is neutral, acidic, or basic. Indicate if each is
hydrophobic or hydrophilic.
16.8 Classify each of the amino acids in problem 16.6 as polar or nonpolar. If
polar, indicate if the R group is neutral, acidic, or basic. Indicate if each is
hydrophobic or hydrophilic.
16.9 Give the name for the amino acid represented by each of the following
abbreviations:
a. Ala
b. Q
c. K
552
口
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 22-44 How can a protein act as a buffer?arrow_forward22-21 Explain why an amino acid cannot exist in an un-ionized form at any pH.arrow_forwardThe pka's of the amino acid asparagine are 2.02 and 8.80. Select the asparagine structure that predominates at pH 10. Structure b Structure a Structure c Structure di NH, NH,arrow_forward
- 3. How is the following amino acid classified? H2N-CHC-OH CH2 C=0 ОН a. nonpolar b. polar neutral c. polar acidic d. polar basicarrow_forwardName each peptide using both the one-letter and the three-letter abbreviations for the names of the component amino acids.arrow_forwardThe isoelectric point (p) of the following amino acid is determined to be 5.97. H2N. OH Which of the following is the dominant form at pH 6.5? Select one: H2N. OH H,N H,N 10arrow_forward
- 4. How is the following amino acid classified? H3Ñ-CHC-o CH2 a. nonpolar b. polar neutral c. polar acidic d. polar basicarrow_forwardpparrow_forward9) A COOH H₂N-C-H CH₂ CH₂ CH₂ CH₂ NH₂ 2.18 Coo H₂N-C-H CH₂ CH₂ CH₂ 8.95 Coo HN-C-H CH₂ CH₂ CH₂ 1 CH₂ NH₂ 16.53 What is this amino acid and the pl? a) This is arginine and the pl is 8.50 b) This is glutamine and the pl is 5.57 c) This is arginine and the pl is 9.62 Coo H₂N-C-H CH₂ 1 CH₂ CH₂ CH₂ NH₂arrow_forward
- Choose from A-F. Two of this amino acid can react with each other (anoxidation reaction) and form a covalent bond. CO- COO COO H3N-C-H H,N-C-H CH2 H2N CH 2 Н-С—он CH2 H2C CH2 CH3 CH3 Choice "A" Choice "B" Choice "C" Coo H,N-C-H H,N-C-H H3N-C-H CH2 CH2 C=CH NH CH2 C-NH | CH SH C-N H Choice "E" Choice "F" Choice "D"arrow_forward1. Draw the structure of the amino acid in a) acidic solution at a pH below the isoelectric point b) basic solution at a pH above the isoelectric point HN-CH-C-O CH, T CH CH, CH,arrow_forwardacids Identify which of the following are a-amino acids. CH₁ H₂N-C-COOH a. CH₁ H b. H₂N-C-CH₂-COOH 1 CH, H c. H₂N-CH₂-C-COOH H 1 d. H₂N-C-COOH CH₂CH,arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning