
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please type all work
Question 6
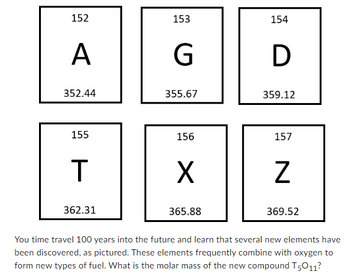
Transcribed Image Text:152
A
352.44
155
T
362.31
153
G
355.67
156
X
365.88
154
D
359.12
157
Z
369.52
You time travel 100 years into the future and learn that several new elements have
been discovered, as pictured. These elements frequently combine with oxygen to
form new types of fuel. What is the molar mass of the new compound T5011?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Mn in MnO4 Mn in Mn2+ Bi in BiO3 Bi in Bi3+ Oxidation Number +2 +3 +5 +6 +7 Drag and drop here Drag and drop here Drag and drop here Drag and drop here Drag and drop here • SUBMIT ANSWER MacBook Air 80 888arrow_forwardBoxes 1-4. box 1 answer choices: plating and the anode, eroding and the anode, eroding and the cathode, plating and the cathode. box 2 answer choices: right or left. box 3 answer choices: right or left. box 4 answer choices: sink of electrons and the (-) terminal, source of electrons and the (+) terminal, sink of electrons and the (+) terminal, or source of electrons and the (-) terminal.arrow_forwardnerbsl@yahoo.com View Help O Find S Replace AaBbCcDc AaBbCcDc AaBbCc AaBbCcD I Normal 1 No Spac. Heading 1 Heading 2 Dictate Ser A Select v Styles Editing Voice Sen 1) What is measured in Volts? a) how many electrons are passing through a system b) how many electrons per second are passing through a system c) difference in potential (pressure) of electrons passing through a system d) total power (ability to do work) of the electrical current 2) What are standard cell potentials, E cell? a) how many Volts this electrochemical cell should produce b) potential of the cell at standard conditions (298K, all aqueous concentrations are 1M, 1 atm.) c) whether the cell is oxidized or reduced d) how many Watts this electrochemical cell should produce 3) What is oxidation? a) oxidation is gain of electrons, oxidation number becomes more negative b) oxidation is gain of electrons, oxidation number becomes more positive c) oxidation is loss of electrons, oxidation number becomes more negative d)…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY