
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
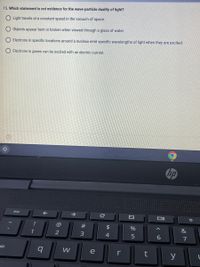
Transcribed Image Text:15. Which statement is not evidence for the wave-particle duality of light?
Light travels at a constant speed in the vacuum of space.
Objects appear bent or broken when viewed through a glass of water.
Electrons in specific locations around a nucleus emit specific wavelengths of light when they are excited.
Electrons in gases can be excited with an electric current.
hp
esc
<>
@
$
&
1
3
4.
7
qE
W
e
r
t
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ( do the two with explanation)arrow_forwardYou will make a graph of your data from the helium spectrum. What purpose will it serve in the data analysis? O It will allow us to calculate the energies of the lines in the helium spectrum based on their wavelengths. O It will allow us to calculate the energies of the lines in the hydrogen spectrum based on their wavelengths. O It will allow us to calculate the wavelengths of the lines in the helium spectrum based on their positions. O It will allow us to calculate the wavelengths of the lines in the hydrogen spectrum based on their positions. O It will allow us to calculate the colors of the lines in the hydrogen spectrum based on their times.arrow_forwardThe energy levels the electron can occupy in the Li2+ ion can be calculated using the energy level equation. A Li2+ ion emitted a photon with a wavelength of 72.9 nm to reach an energy level with n=2. What was the value of n for its initial energy level?arrow_forward
- quizzes/982684 OneNote 101 Chem101 Tophat * USU Tutoring Scholarships :) Morgage Amer Why is light of at least a certain frequency needed to cause the photoelectric effect? O A certain quantity of energy is necessary to eject electrons from a metal A certain number of photons is necessary to eject electrons from a metal A certain temperature is necessary to eject electrons from a metal O A certain number of atoms is necessary to eject electrons from a metal O A certain speed of light is necessary to eject electrons from a metalarrow_forwardWhen electrons pass through a sheet of aluminum, a pattern of high and low intensity is observed. What claim does this experimental evidence support? Electrons are particles. Light is a particle. Light is a wave. Electrons are waves.arrow_forward9. An electron in the hydrogen atom relaxes to the n =2 orbit and 434 nm light is emitted. What was the initial orbit of the electron? A: 5 Me g ou counoearrow_forward
- A hydrogen atom in the n=4 state decays down to the n=2 state. What is the wavelength of light emitted? State your answer in nm. Note: wavelength is a positive number.arrow_forwardIdentify the neutral element represented by this excited-state electron configuration. excited state: 1s²2s²2p63s²3p³ 4s¹ element symbol: Write the full ground-state electron configuration for that element. ground state: FEB 12 tv all A zoomarrow_forwardThe work function (φ) for a metal is the amount of energy needed to eject an electron from the surface of the metal via the photoelectric effect. For nickel φ = 8.25 x 10-19J. Will a photon with a wavelength (λ) of 250.0 nm have enough energy to eject an electron from nickel? If the speed of an electron in the n = 4 energy level of a hydrogen electron is 2.3 x 106 m/s, what is the de Broglie wavelength of this electron in nanometers (the mass of an electron is 9.10938 x 10-31 kg). Note: 1 ? = 1 ?? ∙?2 An F-15 Strike Eagle weighs in at roughly 32,000 kg fully loaded. If an F-15 Eagle is traveling at 3,017 kilometers per hour, what is the de Broglie wavelength associated with the aircraft at this speed in nanometers?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY