
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How would you solve the fifth question (there is only one trial to calculate the molar mass of butane for)?
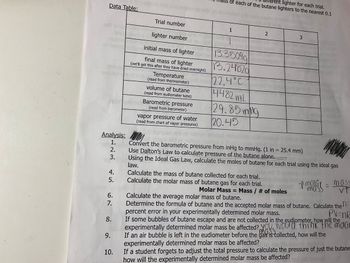
Transcribed Image Text:Data Table:
Analysis:
1.
-
2.
3.
4.
5.
6.
7.
8.
9.
10.
Trial number
lighter number
initial mass of lighter
final mass of lighter
(we'll get this after they have dried overnight)
Temperature
(read from thermometer)
volume of butane
(read from eudiometer tube)
Barometric pressure
(read from barometer)
vapor pressure of water
(read from chart of vapor pressures)
nt lighter for each trial.
ss of each of the butane lighters to the nearest 0.1
1
2
13.35099
13.24820
22.4°C
4482 mL
29.85 ing
20.45
3
AWOWO
Convert the barometric pressure from inHg to mmHg. (1 in = 25.4 mm)
Use Dalton's Law to calculate pressure of the butane alone.
Using the Ideal Gas Law, calculate the moles of butane for each trial using the ideal gas
law.
Calculate the mass of butane collected for each trial.
Calculate the molar mass of butane gas for each trial.
1 molar = mass
mass
Molar Mass= Mass / # of moles
VT
Calculate the average molar mass of butane.
Determine the formula of butane and the accepted molar mass of butane. Calculate the n
percent error in your experimentally determined molar mass.
PV=nR
If some bubbles of butane escape and are not collected in the eudiometer, how will the
experimentally determined molar mass be affected? You would think the malar
If an air bubble is left in the eudiometer before the collected, how will the
experimentally determined molar mass be affected?
If a student forgets to adjust the total pressure to calculate the pressure of just the butane
how will the experimentally determined molar mass be affected?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Practice Exercise 7.8 Calculate the percent composition by mass of potassium in K2CrO4. % potassiumarrow_forwardCalculate number of moles of a substance having 341gram mass and molar mass 568 gram/ molearrow_forward16) What mass % of calcium is in calcium carbonate? a) 9% b) 40% c) 60% d) 100%arrow_forward
- Question down below.arrow_forward1. How many GRAMS of potassium chromate are present in 4.86 moles of this compound ? grams. moles. 2. How many MOLES of potassium chromate are present in 3.02 grams of this compound ?arrow_forwardHow many moles of hydrogen atoms are contained in 0.50 mol of ethyl ether?arrow_forward
- Calculate the molar mass of each compound given below. HINT: We cannot get the molar mass of a compound directly from the periodic table as we did for atomic molar masses. To calculate the molar mass of a compound we add up the atomic molar masses of all atoms in the chemical formula. Can you help me, please?arrow_forwardExplain in detail how you would go about calculating the percent oxygen in magnesium nitrate.arrow_forwardwhat careers study the molar massarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY