
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
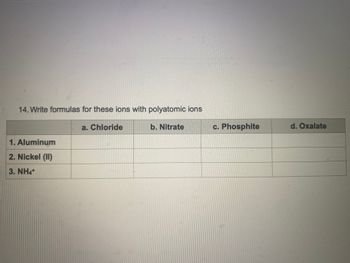
Transcribed Image Text:14. Write formulas for these ions with polyatomic ions
a. Chloride
1. Aluminum
2. Nickel (II)
3. NHạt
b. Nitrate
c. Phosphite
d. Oxalate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q4. A metal ion with a 2+ charge has 24 electrons and forms a compound with an ion that contains 8 protons. a. what is the identity of the metal? b. determine the formula of the compound that is formed?arrow_forwardHow can we write the formulas for the questions?arrow_forwardConsider three isolated ions: Mg2+, 0²- and Na*. Which is true about these ions? A. All of the ions are the same size. B. All of the ions have the same numbers of protons. C. All of the ions have the same electrostatic attraction between protons and electrons. D. All of these ions have the same number of electrons present. E. A and D O C 0 0 0 0 0 UBD w OB OD OE OAarrow_forward
- QUESTION 5 Which one of these compounds is an ionic compound? O a. N2 O b. CO2 O c. Na20 O d. CCI4arrow_forward13. Name or write the formula for each substance. a. Nitric acid has a formula of b. CBR4 is named C. Pb(NO2)2 is named d. Diphosphorus hexaiodide has a formula of e. Gold(III) Oxide has a formula of f. Cas(PO4)2 (aq) is namedarrow_forwardName the following ions S-2 S-1 So32 -2 SO41 HSO31 H2SO4 H2SO3 HSO32 a. doesn't exist b. not an ion C. sulfite d. hydrogen sulfite e. sulfate f. hydrogen sulfate g. sulfide h. suzy qarrow_forward
- 1. Determine the number of protons and electrons in each of the following. Enter your answers numerically separated by a comma. Sr^2+ 2. name the ionic compound a. HgBr2 b. CoCl3 3. name the ionic compound containing a polyatomic ion a. Ba(OH)2 b. NH4Br c. NaBrO4 d.Fe(OH)2arrow_forwardX is an element from group 3A and Y is an element from group 6A what would it's chemical formula be? what is the chemical formula for each group i, ii, iii?arrow_forwardGive the ion symbol for each ion. a. barium ionc. oxidee. lead(IV) b. iron(II)d. ferrous f. cobalt(III)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY