
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
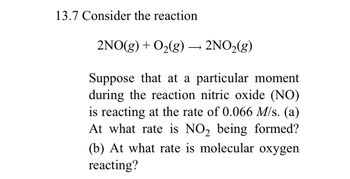
Transcribed Image Text:13.7 Consider the reaction
2NO(g) + O₂(g) → 2NO₂(g)
Suppose that at a particular moment
during the reaction nitric oxide (NO)
is reacting at the rate of 0.066 M/s. (a)
At what rate is NO₂ being formed?
(b) At what rate is molecular oxygen
reacting?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The oxidation of ammonia produces nitrogen and water via the following reaction: 4NH3(g) + 302(g) → 2N2(g) + 6H₂O(A) Suppose the rate of formation of H₂O(A) is 3.0 mol/(Ls). Which of the following statements is true? The rate of consumption of NH3 is 2.0 mol/(L. s). The rate of consumption of O₂ is 2.0 mol/(L. s). The rate of formation of N₂ is 1.3 mol/(Ls). The rate of formation of N₂ is 2.0 mol/(Ls). The rate of consumption of NH3 is 0.50 mol/(L. s).arrow_forwardNitrogen, N2, is reacting at a rate of 75.0 mM/min to produce NH3 in the following reactionN2(g) + 3 H2(g) → 2 NH3(g)What is the rate of change of H2 and what is the rate of change of NH3? Give your answer in mM/min. (Note: mM = millimol/L = 0.001 mol/L)arrow_forwardConsider the following reaction: (a) The rate law for this reaction is first order in NO₂(g) and first order in O3(g). What is the rate law for this reaction? Rate = k [NO₂(g)] [03(g)] Rate = k [NO₂(g)]² [03(g)] Rate = k [NO₂(g)] [03(g)]² O Rate = k [NO₂(g)]² [03(g)]² Rate = k [NO₂(g)] [03(g)]³ Rate = k [NO₂(g)]4 [03(g)] (b) If the rate constant for this reaction at a certain temperature is 97900, what is the reaction rate when [NO₂(g)] = 0.587 M and [03(9)] = 1.40 M? Rate = 2 NO₂(g) + 03(9) → N₂O5(9) + O₂(g) M/s. Rate = (c) What is the reaction rate when the concentration of NO₂(g) is doubled, to 1.17 M while the concentration of O3(g) is 1.40 M? M/Sarrow_forward
- Under certain conditions the rate of this reaction zero order in dinitrogen monoxide with a rate constant of 0.0064 M · s: 2N,0 (g) – 2N, (g) +0, (g) Suppose a 3.0 L flask is charged under these conditions with 400. mmol of dinitrogen monoxide. How much is left 5.0 s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardFor each of the rate laws below, what is the order of the reaction with respect to the hypothetical substances X, Y, and Z? What is the overall order? (For each answer, enter an exact number as an integer or decimal.) (a) rate = k (X][Y][Z]² X Y Overall rate = k (X]/[Y]/² [Z] -1 (b) 1/2 X Y Overall (c) rate = k [Y] X Y Overallarrow_forwardThe overall reaction of ozone reacting to form oxygen has been proposed to occur in a reaction mechanism of: Step 1: O3(g)-→O2(g) + O(g) Step 2: O3(g) + O(g) →202(g) What is the role of O(g) and what is the overall balanced equation? Periodic Table and Datasheet Select one: O a. O(g) is an intermediate; 203(g) + O(g) →302(g) + O(g) O b. O(g) is a catalyst; 203(g) + O(g)→302(g) + O(g) O c. O(g) is an intermediate; 203(g) → 302(g) O d. O(g) is a catalyst; 203(g)→302(g) Which statement best describes general equilibrium?arrow_forward
- A. Consider the following proposed mechanism for the production of chlorine dixoide from chlorine and ozone. Draw a reaction coordinate diagram for this reaction. Label reactants, products, transition states, intermediates, and activation energy on your sketch. Assume the reaction is endothermic. NOTE: CI is chlorine, not Carbon lodide Overall: Cl₂ (g) + 203 (g) → 2 CIO₂ (g) + O₂ (g) Step 1: Cl₂ (g) → 2 Cl (g) fast Step 2: Cl (g) + Os (g) CIO₂ +O fast Step 3: O (g) + O3 (g) → 202 (g) fast Step 4: Cl (g) + O2 (g) → CIO2 (g) slowarrow_forwardGen Chemistry : Please see attached imagearrow_forward(a) Consider the combustion of ammonia, given below: 4 NH3(g) + 5 O2(g) --------> 4 NO(g) + 6 H2O(g) If NH3(g) is decreasing at the rate of 0.130 mol/s, what are the rates of change of O2(g), NO(g), and H2O(g)?O2(g)/t = _____ mol/sarrow_forward
- What is the rate of reaction if the rate of decomposition of dinitrogen pentoxide is 1.648x10 −4 M/s?2 N 2O 5( sol'n) → 4NO 2( sol'n) + O 2( g)arrow_forward9:21 < A) s-¹ B) M-¹s-¹ The reaction, 2 NO(g) + O₂(g) → 2 NO₂(g) has a rate law, rate = k{NO] [O₂]. If the rate of the reaction is 0.60 M/s when [NO] is 1.5 M and [O₂] is 2.0 M, what are the units of the rate constant, k? C) Ms-¹ Question 5 of 20 D) M-²S-¹ E) None of these. 11 Tap here or pull up for additional resources Submitarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY