
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
This problem is (13.11) from a book "

Transcribed Image Text:13.11. Show that the polymer-solvent mixing entropy of Eqn. (13.66) might also be
written in terms of mole fractions as
ASmix
Мx1
X2
-N1 In
1(M 1)x1
- N2 In
М- (М—1)x2,
Кв
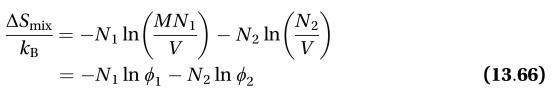
Transcribed Image Text:ASmix
N2
N2 In
V
-N1 In
V
kB
=-N1 ln
N2 In 2
(13.66)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Q.1 Find the solution of following ODE by Laplace transform of the function x( t ) and Ca(t) that satisfies the differential equation and initial conditions. (b) 3 d²x dt² dx - x = 2 dt -2.5 d at x(0) = 1arrow_forwardWhat is the best cubic equations of state being used nowadays in process simulation: Select one: O a. Redlich-Kwong-Soave O b. Peng-Robinson Oc. Van der Waals O d. Redlich-Kwongarrow_forwardMULTIPLE CHOICE -The answer is one of the options below please solve carefully and circle the correct option Please write clear .arrow_forward
- On single-component phase diagrams, the phase rule is given by f = 3 – p. This equation means that if f = 1, the region containing the indicated phases is a line or curve. True Falsearrow_forwardderivation of ideal gas equation for kinetic theory of gas 1.frequency of collision 2.derive the force 3.To determine the pressure of the gas 4.to determine energy associated with ideal gas 5.determine ideal gas equation 6.determine V_rms² from ideal gas Please break each derivation step by step for better understanding ,if possible include diagramarrow_forwardFind Cau/ap), in terms of measurable эр CP-V-T)- Show the mathematical derivation T www.arrow_forward
- In the equilibrium-stage model equations, are K-values and enthalpies counted as variables? Are the equations used to compute these properties counted as equations?arrow_forwardradiationarrow_forwardI need help on figuring out how to use a maxwell relation to find (aS/aV)T fotr the following equation of state. This is the entire questionarrow_forward
- If there are no constraints, how would you conduct a stage-wise distillation column so as to bring its efficiency closer to unity?arrow_forwardPlease use Book: Introduction to Chemical Engineering Thermodynamics 8 edition, 2018 (Smith, J.M., Van Ness, H.C., Abbot, M.M., Swihart.)arrow_forwardIt proposes values for plotting the 6 different viscosity models (Dilatant, Newtonian, Psudoplastic, Plastic Bingham, Psudoplastic Bingham, Dilatant Bingham), all with the help of the formula:All this with the help of matlab. I need help plz i don't realy now how to use this.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The