
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Which isomer would give two signals in its C-NMR spectrum?
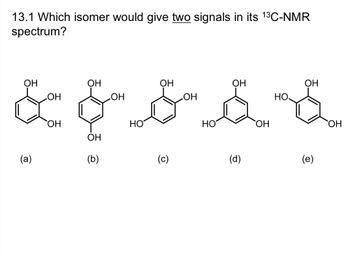
Transcribed Image Text:**Question 13.1**: Which isomer would give two signals in its \(^{13}\text{C-NMR}\) spectrum?
**Isomer Structures**:
(a) **Chemical Structure**: A benzene ring with hydroxyl groups (-OH) at ortho positions (1,2,3-tri-hydroxybenzene).
(b) **Chemical Structure**: A benzene ring with hydroxyl groups in a symmetrical arrangement at positions 1,3,5 (1,3,5-trihydroxybenzene).
(c) **Chemical Structure**: A benzene ring with hydroxyl groups at meta positions (1,2,4-trihydroxybenzene).
(d) **Chemical Structure**: A benzene ring with hydroxyl groups at para positions (1,4-benzoquinone structure).
(e) **Chemical Structure**: A benzene ring with hydroxyl groups at positions 1,2,5 (hydroquinone structure).
**Explanation**:
For \(^{13}\text{C-NMR}\), the number of signals corresponds to the number of unique carbon environments in the molecule. When there are symmetries in the molecule, different carbons in the symmetric positions will give the same signal.
- Isomer (b) will display two signals due to its symmetrical arrangement, with carbons in positions 1,3,5 being equivalent, and carbons in positions 2,4,6 being equivalent.
Expert Solution
arrow_forward
Step 1
NMR spectroscopy is an important tool for the determination of the structure of an organic compound. Carbon-13 NMR spectroscopy detects the energy levels in the nucleus of carbon-13 and hence detects the electronic environment around the carbon nucleus.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many signals will be present in the ¹H NMR spectrum of the molecule below? Do not consider split signals as seperate signals. 0000 6 HC H3C о- CH3 CH3 CH3 CH3arrow_forwardA compound with molecular formula C17H36 exhibits a 1H NMR spectrum with only one signal. How many signals would you expect in the 13C NMR spectrum of this compound? 3 3arrow_forwardA B O || CH3CH₂CH₂COCH 3 CH3CH₂COCH₂CH3 CH3COCH₂CH₂CH3 (a) True/False, all three compounds have the same number of ¹H NMR signals?_ (b) Which compound has the furthest downfield, three hydrogen singlet, (3H, singlet)? (c) Which compound lacks a two hydrogen triplet, (2H,triplet)?_ (d) Which compund has two unique signals that are both three hydrogen triplets, (3H,triplet)?_arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY