
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
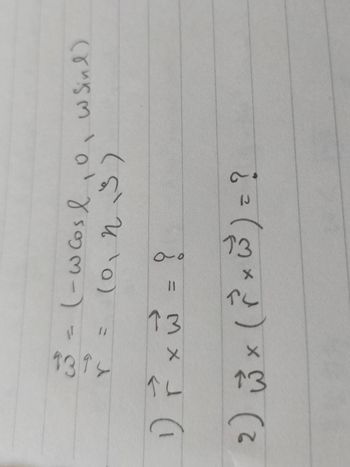
Transcribed Image Text:10
1
(-wcose, o, wsind)
(023)
?
2) ₁ × ( ² × 5³² ) = ?
1392
1 F
11
r x w
الت
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Oil and coal generally contain no measurable 14C. What does this tell us about how long they have been buried?arrow_forwardIn a real or imaginary nucleus of 45X⁹7, (a) how many protons are in the nucleus, (b) how many neutrons are in the nucleus, and (c) how many electrons are in orbit about the nucleus, assuming the atom is electrically neutral? (a) Number (b) Number i (c) Number Units Units Unitsarrow_forwardS Can 6 PAR к Торс K Unit K In x K Moti = Cop K Unit S Spee S Topo S Math Micr eb.kamihq.com/web/viewer.html?source-filepicker&document_identifier=137VZR5BZOVSAIMOA55WU555_CvJ9NacO + 100 P e Interpreting Graphs Answer the questions following the graphs on each side Dietance va. Time 2 7 10 11 12 13 14 Time in soconds 1. From 1 second to 2 seconds, how fast is the object traveling. (Take the difference in distance and divide it by the time in between the 2 distances) 2. Is the object going as fast between 9 and 12 seconds as it is between 1 and 4 seconds? How can you tell? 3. What is the motion of the object between 4 and 6 seconds? acerarrow_forward
- Calculate the standard uncertainty in z if z=Xsinθ using the angle (45.00 ± 0.74) degrees and the value X = (23.60 ± 0.51) z = ___±___arrow_forwardState how many significant figures are proper in the results of the following calculations. (a) (106.7)(6.5)(46.210)(1.01) (b) (25.8)2 (c) (2.9 ✕ 10−19)(3712)arrow_forwardQuestion 12 of 22 Calculate the mass defect of Nitrogen (A = 14, Z = 7). The atomic mass of Nitrogen is 14.00307 u. (Note: The mass of a hydrogen atom is mH = 1.007825 u, and the mass of the neutron is mN = 1.008665 u.) 00 1.21 u 0.53 u 0.25 u 0.11 u zeroarrow_forward
- Calculate the standard uncertainty in z ifz=sinθusing the angle (46.60 ± 0.13) degreesz = __± __arrow_forwardCalculate the standard uncertainty in z ifz=sinθusing the angle (46.60 ± 0.13) degreesz = __± __arrow_forwardBy READING the N vs t graph shown below, determine No & the half-life. N (x10¹ atoms) 120 90. 60. 30. 1 2 3 4 5 6 7 8 9 10 t(y) No = half-life = atomsarrow_forward
- If A =〖yz〗^2 i-3xz^2j +2xyz k, B = 3xi +4zj -xyk and ∅= xyz, findd^2/dxdy [Ax (B.∅)] at P(1, 1, 1)arrow_forwardA rock contains the radioactive isotope ? − 40 that decays with a half-life of 1.3 ??????? ????? and the radioactive isotope ?? − 87 that decays with a half-life of 48.6 ??????? ?????. The rock is 100 ??????? ????? old. The rock currently has 1000 times as many ?? − 87 atoms as there are ? − 40 atoms. (That is, ??? = 1000 × ??.) When the rock first formed it had ? times as many ?? − 87 atoms as ? − 40 atoms. (That is, ???,0 = ? × ??,0.) What is the value of ??arrow_forward2 D -e Physical constants (A) (in m) Bohr Model mv² - h = 6.626 x 10-34 Js; Ke² = 2.307 x 10-28 Jm; m = 9.11 x 10-31 kg. The old Bohr model of the hydrogen atom was based on... (1) the assumption that the electron travels on a circle (A) What is the radius of the orbit with n = 5? (B) What is the speed of the orbit with n = 5? Ke² ra and obeys Newton's second law, and (2) the hypothsis that angular momentum is quantized. For the Bohr model, (1) mvr = n (2) 27 OA: 2.774x10-10 OB: 3.468x10-10 OC: 4.334x10-10 OD: 5.418x10-10 DE: 6.773x10-10 OF: 8.466x10-10 OG: 1.058x109 OH: 1.323x10-9 Submit Answer Tries 0/20arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON