
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Please help me with the proteins and DNA section of the worksheet brliw

Transcribed Image Text:ܝܚܕ ܟܕ ܚ ܥܛܕ
4.
4.
2.
3.
1.
Polysaccharides
2.
3.
4.
DNA
1.
SCH 4U0
NATURAL POLYMERS – INDEPENDENT STUDY
As living things, we are a collection of complex organic molecules. Being made of over 70% water, the
problem of solubility is a serious one. Keep in mind how these organic molecules are structured to
enhance their solubility.
Use pages 124-127 to help you answer these questions in your notes. Be sure to use
complete sentences and diagrams to help summarize relevant information.
Proteins
What is the name of the monomer that all polysaccharides are composed of?
What is the predominant functional group in a polysaccharide?
What type of covalent linkage connects the monomers?
Compare and contrast the structure and function of starch and glycogen.
What is the name of the monomer that all proteins are composed of?
What are the predominant functional groups in proteins?
What type of covalent linkage connects the monomers?
Sketch the two amino acids on page 126. Contrast the polarity of the side chains.
What is the name of the monomer that DNA is composed of?
What is the predominant functional groups in DNA?
The covalent linkage between nucleotides is called a phosphodiester bond. Sketch this linkage.
What type of bond connects the two strands of the DNA double helix?
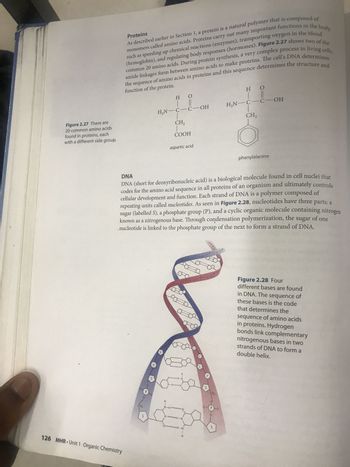
Transcribed Image Text:Figure 2.27 There are
20 common amino acids
found in proteins, each
with a different side group.
Proteins
As described earlier in Section 1, a protein is a natural polymer that is composed of
monomers called amino acids. Proteins carry out many important functions in the
such as speeding up chemical reactions (enzymes), transporting oxygen in the blood
(hemoglobin), and regulating body responses (hormones). Figure 2.27 shows two of the
amide linkages form between amino acids to make proteins. The cell's DNA determines
the sequence of amino acids in proteins and this sequence determines the structure and
function of the protein.
126 MHR Unit 1 Organic Chemistry
0
CH₂
S
H
O
| ||
H₂N-C-C-OH
DNA
DNA (short for deoxyribonucleic acid) is a biological molecule found in cell nuclei that
codes for the amino acid sequence in all proteins of an organism and ultimately controls
cellular development and function. Each strand of DNA is a polymer composed of
repeating units called nucleotides. As seen in Figure 2.28, nucleotides have three
sugar (labelled S), a phosphate group (P), and a cyclic organic molecule containing nitrogen
known as a nitrogenous base. Through condensation polymerization, the
nucleotide is linked to the phosphate group of the next to form a strand of DNA.
parts: a
sugar
of one
S
S
CH₂
COOH
H
aspartic acid
0.***..H-N
N-HN
D
N-H*******0
N******H-N
O*******H-N
1
H
S
CH₂
O=(P
Η Ο
1 11
H₂N-C-C-OH
I
CH₂
S
phenylalanine
Figure 2.28 Four
different bases are found
in DNA. The sequence of
these bases is the code
that determines the
sequence of amino acids
in proteins. Hydrogen
bonds link complementary
nitrogenous bases in two
strands of DNA to form a
double helix.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- This question has been posted previously with parts A B and C already answered. Please focus on only D, E and F Provided is the question alongside with the added materials next to it. Thank youarrow_forwardPlease Write at your own words -Explain this How we can treatment for the genetic disorders by using gene therapy? Please explain at your own words, please.arrow_forwardi need help with these questions! please and thank you a. When would you use Edman degradation method and mass spectrometry inprotein sequencing experiment?b. What is the biochemical information you acquire from amino acid sequence?Give three examples.c. You are say buying a pure enzyme for your experiment. Would you choose an enzyme with high activity or high specific activity? Why/arrow_forward
- K Ć Chrome File Edit View History Bookmarks. Profiles Tab Window Help Content X Biol 1406 X Content X Begin: Test X A Tutorial for X acconline.austincc.edu/ultra/courses/_891351_1/cl/outline A Unit 3 Ove X A Unit 3 Ove X ☎ D)) 33% Content Choose all the types of mutations which may result in a frameshift mutation: nucleotide insertion nucleotide substitution nucleotide deletion X G We G Trar Qarrow_forwardNeed help on question 3, please. The drop-down answer choices are in the image attached below.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON