
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
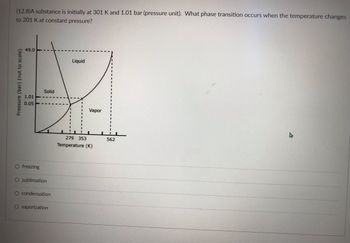
Transcribed Image Text:### Phase Transition in a Substance
**Question:**
A substance is initially at 301 K and 1.01 bar (pressure unit). What phase transition occurs when the temperature changes to 201 K at constant pressure?
**Graph Explanation:**
The graph represents the phase diagram of a substance, showcasing the phases it will be in under different temperatures and pressure conditions. The y-axis represents the pressure in bar (not to scale) and the x-axis represents the temperature in Kelvin (K).
- **Regions:**
- The region labeled "Solid" represents the solid phase.
- The region labeled "Liquid" represents the liquid phase.
- The region labeled "Vapor" represents the vapor (gaseous) phase.
**Key Points:**
- The triple point is where all three phases coexist in equilibrium.
- The graph exhibits the boundaries between different phases.
**Current State:**
The substance is initially at 301 K and 1.01 bar, which is in the "Liquid" region.
**Temperature Change:**
When the temperature is lowered to 201 K while maintaining a constant pressure of 1.01 bar, the substance moves into the "Solid" region.
**Phase Transition:**
The phase transition that occurs is indicated by the movement from the liquid region to the solid region on the phase diagram.
**Multiple Choice Answer Options:**
- Freezing
- Sublimation
- Condensation
- Vaporization
**Correct Answer:**
- **Freezing**: The substance transitions from a liquid phase to a solid phase at constant pressure when the temperature is lowered from 301 K to 201 K.
### Educational Content: Understanding Phase Transitions
In this example, the process of freezing is explained using a phase diagram. The transition occurs due to a decrease in temperature while maintaining a constant pressure, leading to a change in the phase from liquid to solid. This is a fundamental concept in thermodynamics and is critical in understanding material properties and their applications in various fields such as chemistry, physics, and engineering.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL.. solution 2.0 g of hydrobromic acid (HBr) dissolved in 250. mL of water 2.0 g of glucose (CHO) dissolved in 250. mL of water 2.0 g of potassium nitrate (KND3) dissolved in 250 ml of water. 250, mL of pure water freezing point (choose one) (choose one) (choose one) (choose one) boiling point (choose one) - (choose one) (choose one) (choose one) Aarrow_forwardIs this correct?arrow_forward6. A substance has the following properties; Boiling Point = 120°C, Freezing Point = -20°C, specific heat as a gas, liquid and solid, 0.8 cal/g-C°, 3 cal/g-C° and 2 cal/g-C°, respectively. Heat of fusion=200 cal/g and heat of vaporization=800 cal/g. How much heat must be added in changing 100g of this substance from -40°C to 180°C?arrow_forward
- Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 2.2 g of ethylene glycol (C₂H602) dissolved in 200. mL of water 2.2 g of sucrose (C12H22011) dissolved in 200. mL of water 2.2 g of potassium nitrate (KNO3) dissolved in 200. mL of water 200. mL of pure water freezing point (choose one) (choose one) (choose one) (choose one) X boiling point (choose one) (choose one) O (choose one) (choose one)arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 6.0 g of ethylene glycol (C₂H6O2) dissolved in 200. mL of water 6.0 g of potassium sulfate (K₂SO4) dissolved in 200. mL of water 6.0 g of sucrose (C12H22011) dissolved in 200. mL of water 200. mL of pure water freezing point ✓ (choose one) 1(lowest) 2 3 4(highest) (choose one) X boiling point (choose one) (choose one) (choose one) (choose one) Śarrow_forward3. It is a quantity of heat need to convert a mole of liquid into gas phase at a specified temperature? a. molar enthalpy of vaporization b. molar enthalpy of boiling c. molar enthalpy of freezing d. molar enthalpy of evaporationarrow_forward
- The following information is given for benzene, C6H6, at 1atm: AHvap(80.1 °C) = 30.7 kJ/mol boiling point = 80.1 °C specific heat liquid = 1.74 J/g°C At a pressure of 1 atm, boiling point of 80.1 °C. kJ of heat are needed to vaporize a 40.2 g sample of liquid benzene at its normalarrow_forwardPentane C5H12 boils at 360C at 1 atmosphere pressure. What is the molar heat of vaporization in kJ/mol if the vapor pressure of pentane at 250C is 505 torr?arrow_forward- Suppose a small sample of pure X is held at -182. °C and 0.8 atm. What will be the state of the sample (solid, liquid, or gas)? - Suppose the temperature is held constant at -182. °C but the pressure is increased by 0.3 atm. What will happen to the sample (nothing, it will melt, it will freeze, it will boil, it will condense, it will sublime, or it will deposit)? - Suppose, on the other hand, the pressure is held constant at 0.8 atm but the temperature is increased by 178.°C. What will happen to the sample (nothing, it will melt, it will freeze, it will boil, it will condense, it will sublime, or it will deposit)? This all part of one questionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY