
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%

Transcribed Image Text:12.29. Mixtures of methanol and 1-octanol are prepared, and their temperature is
raised to a level above the boiling point of methanol but below that of 1-octanol.
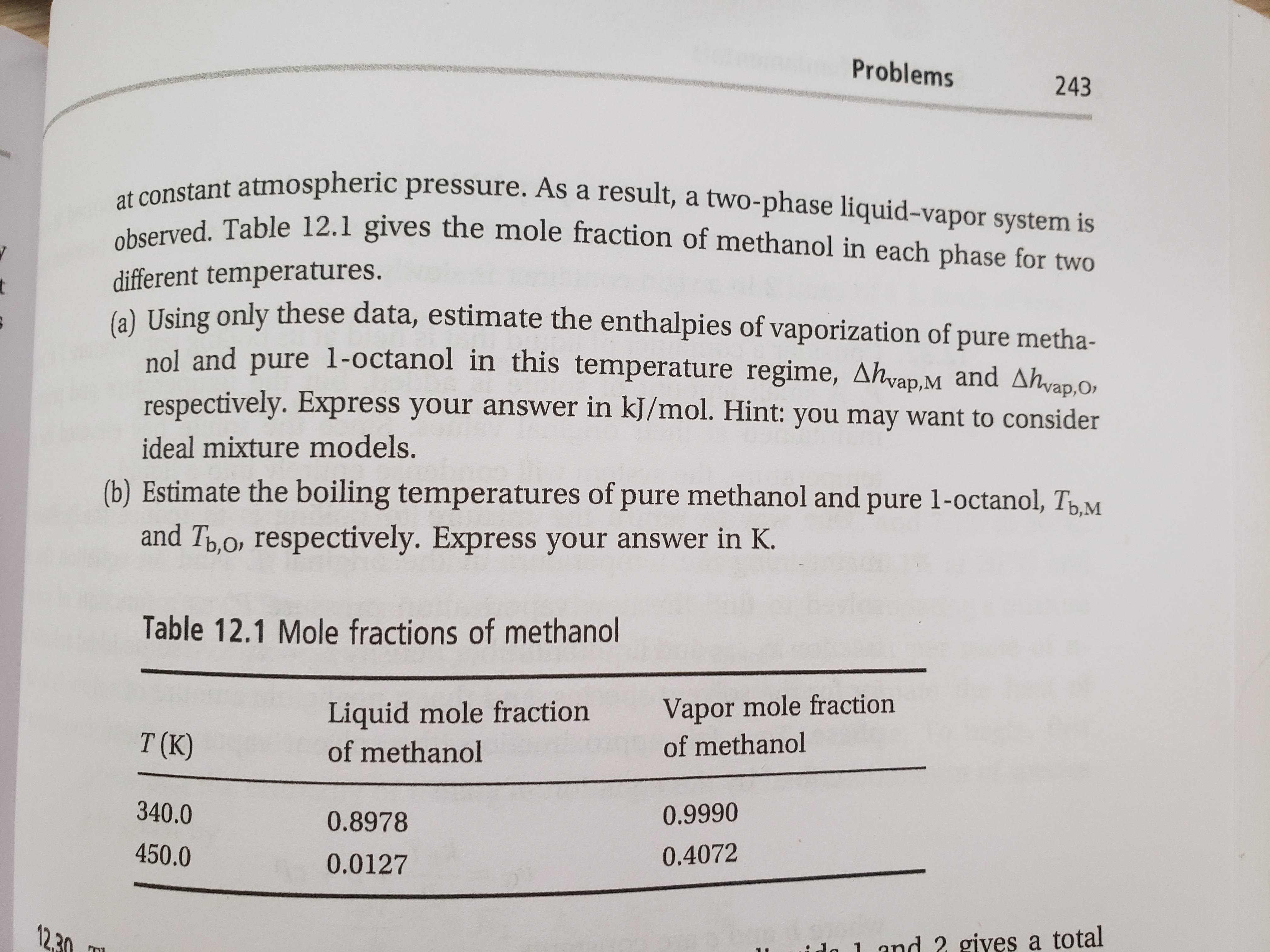
Transcribed Image Text:observed. Table 12.1 gives the mole fraction of methanol in each phase for two
at constant atmospheric pressure. As a result, a two-phase liquid-vapor system is
Problems
243
otant atmospheric pressure. As a result, a two-phase liquid-vapor system is
different temperatures.
6) Using only these data, estimate the enthalpies of vaporization of pure metha-
t
nol and pure 1-octanol in this temperature regime, Ahvap.M and Ahvap.O,
respectively. Express your answer in kJ/mol. Hint: you may want to consider
ideal mixture models.
(b) Estimate the boiling temperatures of pure methanol and pure 1-octanol, TpM
and Tp,o, respectively. Express your answer in K.
Table 12.1 Mole fractions of methanol
Liquid mole fraction
of methanol
Vapor mole fraction
of methanol
T (K)
340.0
0.9990
0.8978
450.0
0.4072
0.0127
12,30
ido 1 and 2 gives a total
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 6 images

Knowledge Booster
Similar questions
- Ideal mixtures What is the boiling point of compound B? Which is more volatile, compound A or compound B? Suppose you mix 1.0 mole of compound A and 4.0 moles of compound B at room temperature (with no air), then heat it. At temperature will the first vapor appear? For the mixture in #3, what will be the composition (mole fraction) in the first vapor? If you have an initial liquid with a mole fraction of A of 0.1, how many theoretical plates are necessary to prepare a mixture which is at least 50% (mole percent) of A? If you have an initial liquid with a mole fraction of A of 0.1, how many theoretical plates are necessary to prepare a mixture which is at least 50% (mole percent) of C?arrow_forwardAbsolute (100%) ethanol is produced from 95% ethanol and 5% water using the Keyes distillation process. Another component, benzene, is added to lower the instability of the alcohol. With these conditions, the product from the top of the distiller is a constant-boiling mixture of 18.5% ethanol, 7.4% H₂O, and 74.1% benzene, as shown here: 95% ethanol 5% water Benzene Distillation tower P(absolute ethanol) = 0.785 g/cm³ P(benzene) = 0.872 g/cm³ 74.1% benzene 18.5% ethanol 7.4% water 100% ethanol Calculate the volume of benzene that should be fed to the still to produce 250 L of absolute ethanol, using this data:arrow_forwardMolecular bromine is 24 percent dissociated at 1600 K and 1.00 bar in the equilibrium Br₂(g) ⇒ 2Br(g). Calculate (a) Kp, (b) A,Gº, (c) K₂ at 2000 K given that A₁HⓇ = +112 kJ mol-¹ over the temperature range.arrow_forward
- 3arrow_forwardUnable how to proceed with a partial pressure problem (For Practice), if anyone could shed light on how to proceed! Thank you so much, I greatily appreciate the helparrow_forwarddetermine the solubility of N2 in water exposed to air at 25°c if the atmospheric pressure is 1.2 bar. assume that the mole fraction of nitrogen is 0.78 in air and the henry's law constant for nitrogen in water at this temperature is 6.1x10^-4 mol/L^-1 bar^-1arrow_forward
- 23. The volumetric diffusivity of a binary mixture is 0.934 ft/hr at 30°C and 2 atm. The molal diffusivity for a similar mixture is a, 4.688 x 10 0.05 lb mole/ft-hr. c. 2.344 x 10 -3 d. none of thesearrow_forwardMethanol (CH3OH) has been suggested as a fuel to replace gasoline. Write a balanced equation for the combustion of methanol, find ΔH °rxn, and determine the mass of carbon dioxide emitted per kJ of heat produced. Use the information from the previous exercise to calculate the same quantity for octane, C8H18. How does methanol compare to octane with respect toglobal warming?arrow_forwardEVALUATION OF MASS DIFFUSIVITY Find the diffusivity of CO, in N2 at 30°C and 740 mm Hg. Find the diffusivity of benzene vapor in air at 600C and 120 kPa Find the diffusivity of isopropyl alcohol in liquid water at 45°Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The