Question
thumb_up100%
1,2 only need answer not explained
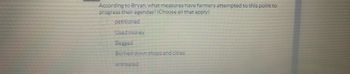
Transcribed Image Text:According to Bryan, what measures have farmers attempted to this point to
progress their agendas? (Choose all that apply)
petitioned
Used money
Begged
Burned down shops and cities
entreated
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps
