Question
The two peaks for the molybdenum spectra of Figure are characteristic spectral lines for the molybdenum element. What is the minimum potential difference needed to accelerate electrons in an x-ray tube to produce both of these lines?
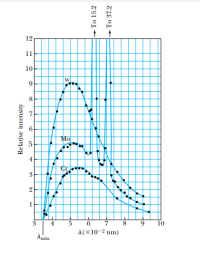
Transcribed Image Text:12
Mo
1
3
9
10
A(X10-2 nm)
Amin
Relative intensity
4,
+ To 15.2
+ To 37.2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Please type instead of hand writtingarrow_forwardThe characteristic K, and K, lines for chromium have wavelengths of 0.229 nm and 0.208 nm, respectively. What is the ratio of the energy difference between the levels in chromium involved in the production of these two lines? ΔΕ. ΔΕ AEarrow_forwardThe work function of a certain metal is found to be 1.82 eV. What is the stopping potential required to to stop the current in a photoelectric effect experiment if the wavelength of the incident light is 413 nm. Round your answer to 3 significant figures. Add your answerarrow_forward
- Light of frequency 12x1015 Hz shines on to clean caesium metal. What is the maximum kinetic energy of the electrons emitted ? The work function of caesium is 1.5 x 10-19 J and the Planck constant h = 6.63 x 10-34 J s. Round off the answer to 2 decimal places and write answers as power of 10.arrow_forwardThe work function of a certain metal is found to be 1.82 ev. What is the stopping potential required to to stop the current in a photoelectric effect experiment if the wavelength of the incident light is 452 nm. Round your answer to 3 significant figures. Add your answerarrow_forwardWhat is the wavelength of an electron of energy 2.0 keV ?arrow_forward
- Font Paragraph Styles Voice Sensitivity 5. The dispersion relation for propagating waves is the equation w = w(k) giving the angular frequency as a function of the wavenumber. A free-electron will have the energy-momentum relation E = 2m Use this relation, in conjunction with Planck's equation and De Broglie's equation, to determine the quantum mechanical dispersion relation for the wave associated with a free electron. S GeneralVAll Employees (unrestricted) 2 Accessibility: Good to go D. Focus words 23 ain Coparrow_forwardWhat is the stopping potential, in volts, of a cathode ray tube that has been illuminated by 209 nm ultraviolet light, and that is made of a metal that has a work function of 3.95 eV (electronvolts)?arrow_forwardNeeds Complete typed solution with 100 % accuracy. Don't use chat gpt or ai i definitely upvote you.arrow_forward
arrow_back_ios
arrow_forward_ios