
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:O
>>
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-liG1Sp4C1qHE9vg-2-exSXq493r_tJ2gZs0a-xkRSR8a
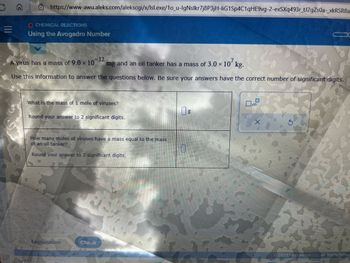
O CHEMICAL REACTIONS
Using the Avogadro Number
-12
A virus has a mass of 9.0 x 10 mg and an oil tanker has a mass of 3.0 × 107 kg.
Use this information to answer the questions below. Be sure your answers have the correct number of significant digits.
What is the mass of 1 mole of viruses?
Round your answer to 2 significant digits.
How many moles of viruses have a mass equal to the mass
of an oil tanker?
Round your answer to 2 significant digits.
Explanation
Check
0
S
x10
S
1
© 2023 PicGraw Hili LLC. All Rights Reserv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The chemical formula for barium sulfide is BaS. A chemist determined by measurements that 0.030 moles of barium sulfide participate in a chemical reaction. Calculate the mass of barium sulfide that participates. Round your answer to 2 significant digits. garrow_forwardGaseous butane (CH₂(CH₂)₂CH3) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 49. g of butane is mixed with 200. g of oxygen. Calculate the minimum mass of butane that could be left over by the chemical reaction. Round your answer to 2 significant digits. Og 0 Xarrow_forwardChemistry A chemist prepares a solution of potassium iodide (KI) by weighing out 145. g of potassium iodide into a 100 ml. volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/dl. of the chemist's potassium iodide solution. Round your answer to 3 significant digits. 02arrow_forward
- GenAlex Medical, a leading manufacturer of medical laboratory equipment, is designing a new automated system that can detect therapeutic levels of dissolved digoxin (1.5 to 2.0 ng/ml.), using a blood sample that is as small as 25. pl.. Calculate the minimum mass in nanograms of digoxin that the new system must be able to detect. Round your answer to 2 significant digits. 0.2arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 20.94 cm x 80 cm - 93. g ml. -* 19. mL - 946.2 mol 31.5 L - 0 cm 2 mol 0.9 Xarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.arrow_forward
- Use mass density to find volume (picture is included)arrow_forwardA chemist weighed out 7.2g of mercury. Calculate the number of moles of mercury she weighed out. Be sure your answer has the correct number of significant digits.arrow_forwardAmmonium phosphate ((NH₂),PO4) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H3PO4) with ammonia (NH3). What mass of ammonium phosphate is produced by the reaction of 1.8 g of ammonia? Be sure your answer has the correct number of significant digits. 7. g 0 10 Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY