
Chemistry for Engineering Students
3rd Edition
ISBN: 9781285199023
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
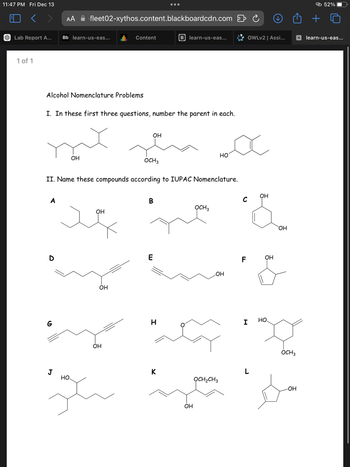
Transcribed Image Text:11:47 PM Fri Dec 13
<
AA
...
-fleet02-xythos.content.blackboardcdn.com ⇓ Ć
Lab Report A...
Bb learn-us-eas...
Content
B learn-us-eas...
1 of 1
Alcohol Nomenclature Problems
I. In these first three questions, number the parent in each.
OH
OH
OCH3
HO
II. Name these compounds according to IUPAC Nomenclature.
A
B
OH
D
G
J
HO
OH
OH
E
ළා 52%
OWLv2 | Assi...
learn-us-eas...
OH
C
OCH3
F
OH
LOH
H
I
HO
OH
OCH3
K
OCH2CH3
-OH
HP
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 15 images

Knowledge Booster
Similar questions
- A group of students compiled the data shown in data table 1 below. What is the exact calculated order for H+(aq) to 2 decimal places? Exp. # [IO3-]0 (M) [I-]0 (M) [H+]0 (M) Time (s) 1 0.005 0.05 2 x 10-5 22.12 2 0.010 0.05 2 x 10-5 86.84 3 0.005 0.10 2 x 10-5 5.35 4 0.005 0.05 4 x 10-5 2.65 Question 6 options: 2.82 -2.51 3.34 2.71 -2.58 3.12 2.91 3.49 3.41 3.23 3.06 2.64arrow_forwardA group of students compiled the data shown in data table 1 below. What is the exact calculated order for IO3-(aq) to 2 decimal places? Exp. # [IO3-]0 (M) [I-]0 (M) [H+]0 (M) Time (s) 1 0.005 0.05 2 x 10-5 22.12 2 0.010 0.05 2 x 10-5 86.84 3 0.005 0.10 2 x 10-5 5.35 4 0.005 0.05 4 x 10-5 2.65 Question 4 options: 2.44 -2.49 1.81 2.09 -1.72 -2.13 -2.36 1.49 2.24 -1.97 -1.53 1.65arrow_forward! 9 a alt ← Z If you live to be approximately 83 years old, how many hours long was your life? Assume a year is exactly 365 days. @ 2 W S →> X #m 3 e d C с $ 4 r f % 5 V t g Oll A 6 DELL b y h & 7 O n u j * 00 O m i k ( 9 < 4 O alt ) O 1 P ◆ ctrl : - ; { [ ? 1 +11 = I } bac 1arrow_forward
- Sign - 12 Helvetica - x apprch. +inf y apprch. -inf So A bacteria triples every second. How much bacteria will there be after 2 seconds, if we began with 2,000 bacteria. The half life of Rutherfordium is 13 hours. This means the amount of Rutherfordium halves everyarrow_forwardQd 105.arrow_forwardhelparrow_forward
- A 2.5 L tank can withstand a maximum pressure of 800 kPa. Calculate the temperature that the tank will explode, given the tank has a pressure of 180 kPa at 25°C. H Be Na Mg K Rb Sr Ba Fr Ra Sc Ti 57-71 Lanthanide La Ce above 67.05 K Hf below 800.2 K above 1546.8 K above 135.6 K O below 970.4 K Viet va above 1267.2 K 23 24 25 26 27 Cr Mn above 1324.4 K Periodic Table of the Elements VID 75 Symbol Abaline Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Tra Fe Co Ni Cu Mesa 13 29 30 31 Pt Au Hg 13 HA Re 89-103 104 106 112 118 114 Rf Db Sg Bh Hs Mt Ds Rg Cn Uut "FI Uup "Lv Uus Uuo Nawal B 33 Zn Ga Ge As 14 Ativice "Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Series W Moham www.m MINN N Bas 16 VIA 34 Lavand 17 VIA 7A 35 Se Br 71 Pr Nd Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu as 18 VELA BA He Ne Xe At Rn 36 Krarrow_forwardWhat is the expected throughput if Ra =67packets/minute and Rt = 109packets/minutearrow_forwardhelp a girl out?arrow_forward
- what is the concentration of the unknown sample using serial diluitions: 0.05M 1.7472nm, 0.025M 0.8569nm, 0.0125M 0.4167nm, 0.00625M 0.1958nm the epsilon value is 0.0101 and l is 1 cmarrow_forwardcame the molecule: ✓ Saved 0 Harrow_forwardrul CH10 por O Fle| Csersuse/DesitcoCH100020Cecy/futorial-ManuaOCHTO02 paf eD MsA of I8 Q Page vw ARd soud V Dra V Highlight Era 7. What percentage of a material will persist after 60 minutes if it's half-life is 30 minutes? 8 Sodium 24arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning