
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:11:41 Sun, Dec 4 M.
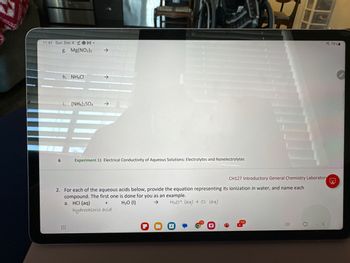
g. Mg(NO₂)2
6
h. NH4Cl
i. (NH4)2SO4
→>
Experiment 11 Electrical Conductivity of Aqueous Solutions: Electrolytes and Nonelectrolytes
2. For each of the aqueous acids below, provide the equation representing
compound. The first one is done for you as an example.
a. HCI (aq)
H₂O (1)
H30+ (aq) + Cl- (aq)
hydrochloric acid
CH127 Introductory General Chemistry Laboratory
its ionization in water, and name each
|||
C
<
79%
X
Transcribed Image Text:11:40 Sun, Dec 4 M.
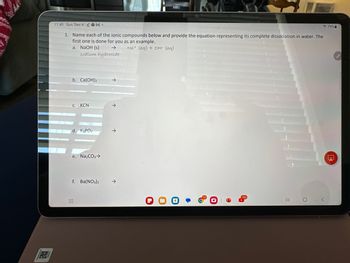
1. Name each of the ionic compounds below and provide the equation representing its complete dissociation in water. The
first one is done for you as an example.
Na+ (aq) + OH (aq)
a. NaOH (s) →
sodium hydroxide
b. Ca(OH)2
C. KCN
d. K3PO4
e. Na₂CO3 →
f. Ba(NO3)2
→
→
163
|||
O
<
79%
(X)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sodium hydrogen carbonate NaHCO3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl, which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq)+NaHCO3(aq)→NaCl(aq)+H2O(l)+CO2(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.mL of a 0.089M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl? Be sure your answer has the correct number of significant digits.arrow_forward1. a) Write the formula for the conjugate base of HPO4^-2 b) Write the formula for the conjugate acid of NH3arrow_forwardI have 3.025 lb H2SO4 and 200 gal of water. How many molecules of sulfuric acid do I have? What is the pH? All hydrogen atoms convert to hydronium ions. 1 oz= 28.35 g 1 gall=3.785 Larrow_forward
- General Chemistry 4th Edition McQuarrie Rock Gallogly ● V = University Science Books presented by Macmillan Learning How many milliliters of 11.5 M HCl(aq) are needed to prepare 400.0 mL of 1.00 M HCl(aq)? mL Publisher: Universi Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemistry Temps rise A Bő 2/arrow_forwardSuppose a 0.031M aqueous solution of sulfuric acid (H₂SO4) is prepared. Calculate the equilibrium molarity of SO4. You'll find information on the properties of sulfuric acid in the ALEKS Data resource. Round your answer to 2 significant digits. IM S P Xarrow_forward3. Maalox, an over-the-counter antacid, contains aluminum hydroxide, Al(OH)3, and magnesium hydroxide, Mg(OH)2. Write balanced equations for the reaction of both with stomach acid (HCI).arrow_forward
- Write a balanced equation for the following acid reactions: Ensure you balance both sides of the equation! Indicate if the product is s, l, g or aq.a. H2SO4 (aq) + Sr(OH)2 (s) →b. H3PO4 (aq) + K2CO3 (s) →c. HCl (aq) + Al(s) →arrow_forwardGive the correct chemical formulas of the products of an acid-base neutralization between H2SO4 and Al(OH)3. (Numbers will be assumed to be subscripts in your answer.) Do not try to enter any physical states or coefficients in the answer boxes. The order in which you list the products does not matter. Formula of one product (do not include the coefficient): Answer Formula of other product (do not include the coefficient): Answer What is the biggest coefficient in the balanced chemical equation for the reaction? (Report a single digit for your answer.) Answerarrow_forwardFive grams of vinegar solution was given to you to analyze for the percent acetic acid present in the vinegar. After the titration, 0.0036 moles of NaOH was used to neutralize the acetic acid solution. Calculate the percent by mass of CH3COOH in the vinegar. O 43.2% O None of the other choices is correct O 86 4% O 34.56 %arrow_forward
- Predict the products (if any) that will be formed by the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. a.) Al(s) + HCl(aq) → b.) 2 HClO₄(aq) + Co(s) → c.) Ca(s) + LiCl(aq) → d.) FeSO₄(aq) + Zn(s) →arrow_forwardSodium hydrogen carbonate (NaHCO,), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains to0 much hydrochloric acid (HCl), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO, neutralizes excess HCl through this reaction: HCl(aq) + NaHCO3(aq) → NaCl(aq) + H,O(1) + CO,g) The CO, gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 150. mL of a 0.064 M HCl solution. What mass of NaHCO, would she need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits. x10 Submit Assig bl)arrow_forwardWrite out these balanced equations (include physical states): Solid iron(II) hydroxide decomposes to form solid iron(II) oxide and liquid water. Solutions of silver nitrate and aluminum iodide are mixed together, forming solid silver iodide and aqueous aluminum nitrate. When calcium metal is placed in water, hydrogen gas bubbles out, leaving a highly alkaline (basic) solution. Write the balanced chemical equation for this reaction. Phases are optional. Do not write an ionic equation (i.e., the answer should not show any charges).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY