
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
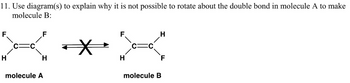
Transcribed Image Text:11. Use diagram(s) to explain why it is not possible to rotate about the double bond in molecule A to make
molecule B:
H
molecule A
molecule B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 40 Assuming a molecular orbital diagram similar to that of O2, how many unpaired electrons are present in N₂*? Oa.2 Ob.4 Oc1 d. 3 Oe.oarrow_forwardH. -CEN How many sigma bonds are in the molecule below? 19 6 9.arrow_forward-Determine electron geometry AND molecular geometry/shape for each -Which molecule (excluding #8) will have the highest boiling point? Which will have the lowest boiling point? 1. CF4 2. NH3 3.H₂S 4. PCI, 5. SO₂ 6. SO3 7. Cl₂ 8. CaCl₂arrow_forward
- How many "central" atoms are part of ethylene glycol, a component of antifreeze? What are teh molecular geometries of each central atom? Is the molecule polar or non-polar? Why?arrow_forward11:05 1 3. Flip to the last page and the table of VSEPR geometries. If a molecule has five electron pairs arranged around the central atom, how many possible molecular shapes could it have? 4. Using the VB definition of sigma and pi bonded electrons to defend your answer, explain why chlorine does not have pi-bonded electrons.arrow_forward52. Determine whether each molecule is polar or nonpolar. SiCl4 CF 2Cl2 SeF6 IF5 ذن فن b. d.arrow_forward
- 10. Answer these 5 questions for the following: 1) Lewis Structure (include all resonance structures) 2) Label any formal charges 3) Name the electron geometry 4) Name the molecular shape 5) Name the polarity а. Bro,1 b. CO,2 c. TeF. d. XeCl4arrow_forwardPlease answer question #9arrow_forwardIdentify the CIS bondarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY