
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
p89#11. the work required to compare a gas reversibly according to pV1.30=c is 67,790J, if there is no flow. Determine change of internal energy and Q if the gas is (a)air,(b) methane. for methane,k=1.321,R=518.45J/kgm.K, Cv=1.6187, Cp=2.1377kJ/kgm.K
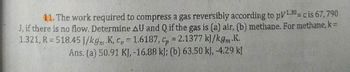
Transcribed Image Text:11. The work required to compress a gas reversibly according to p/130= c is 67, 790
J, if there is no flow. Determine AU and Q if the gas is (a) air, (b) methane. For methane, k =
1.321, R=518.45 J/kgm K, C = 1.6187, Cp = 2.1377 kJ/kgm.K.
Ans. (a) 50.91 KJ, -16.88 kJ; (b) 63.50 kJ, -4.29 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- One-quarter Ibmol of oxygen gas (O₂) undergoes a process from p₁ = 20 lb/in², T₁ = 500°R to p₂ = 150 lb/in². For the process W= -500 Btu and Q = -140.0 Btu. Assume the oxygen behaves as an ideal gas. Determine T2, in "R, and the change in entropy. in Btu/°R. Step 1 Determine T₂, in °R. Your answer is correct. T₂- 78862 Hint Step 2 * Your answer is incorrect. A$12 Determine the change in entropy, in Btu/°R. °R i 0.1968 eTextbook and Media Btu/ºR Attempts: 1 of 4 usedarrow_forwardt = 135°C, h = 567.69 KJ/kgarrow_forwardOne-quarter lbmol of oxygen gas (O2) undergoes a process from p1 = 20 lbf/in2, T1 = 500oR to p2 = 150 lbf/in2. For the process W = -500 Btu and Q = -240.0 Btu. Assume the oxygen behaves as an ideal gas. Determine T2, in oR, and the change in entropy, in Btu/oR.arrow_forward
- Solvearrow_forwardOne-quarter Ibmol of oxygen gas (O₂) undergoes a process from p₁ = 20 lbf/in², T₁ = 500°R to p2 = 150 lbf/in². For the process W = -500 Btu and Q = -177.5 Btu. Assume the oxygen behaves as an ideal gas. Determine T2, in °R, and the change in entropy, in Btu/°R.arrow_forwardTopic: Ideal gas process Instructions: Answer the following review questions and show complete solutions. *please write legibly. Thank youarrow_forward
- p = 0.300 MPa, u = 2543.6 KJ/kgarrow_forwardOne-quarter lbmol of oxygen gas (O2) undergoes a process from p1 = 20 lbf/in2, T1 = 500oR to p2 = 150 lbf/in2. For the process W = -500 Btu and Q = -140.0 Btu. Assume the oxygen behaves as an ideal gas. Determine T2, in oR, and the change in entropy, in Btu/oR.arrow_forwardOne-quarter Ibmol of oxygen gas (O2) undergoes a process from p1 = 20 Ib/in?, T1 = 500°R to p2 = 150 lb;/in?. For the process W = -500 Btu and Q = -127.5 Btu. Assume the oxygen behaves as an ideal gas. Determine T2, in °R, and the change in entropy, in Btu/°R. Step 1 Determine T2, in °R. T2 = °R Save for Later Attempts: 0 of 1 used Submit Answer Step 2 The parts of this question must be completed in order. This part will be available when you complete the part above.arrow_forward
- Q6: n moles of CH4, considered as an ideal gas, are taken through the following thermodynamic cycle: A→B: isovolumetric transformation from pressure PA and volume VA to pressure PB=2 PA B→C: isobaric expansion from volume VA to volume VC=3 VA C→D: isovolumetric transformation down to pressure PD=PA D→A: isobaric compression a. Sketch the (ABCD) cycle on a P-V diagram and determine all the unknown pressures, volumes and temperatures at points A, B, C and D in terms of n, R, PA and VA. b. Determine the net mechanical work done by the gas over the entire cycle. c. Determine the heat absorbed by the gas over each leg of the cycle.arrow_forward3. Ideal air (mass =lkg) in a piston-cylinder assembly undergoes two reversible processes in series from state 1, where T;=290K and Pi= 1bar. The gas constant is 8.314 (kJ/kmol.K). Process 1-2: compression to P3=5 bar in a polytropic process with n=1.19 Process 2-3: expansion in an adiabatic process to P3=1bar Determine the temperature at state 2 and 3 in (K) The total work and heat transfer. Plot on TS diagram.arrow_forwardUsing the appropriate tables, determine the specific entropy change in kJ/(kg K) between the states indicated. a)Water, P1 = 10 MPa, T1 = 400°C, P2 =10 MPa, T2 = 100°C b)R134a, h1 =111.44 kJ/kg, T1 =40°C, saturated steam P2 =5 bar. c) Ideal gas air, T1 =7°C, P1 = 2 bar, T2 =327°C, P2 = 1 bar.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY