11. Complete the chart. **You should not have the same answer for all the IMF in the last column** Molecular Formula Lewis Structure(1) Polarity of Molecule Justify your Polarity of Bond. Justify your answer. What type of Intermolecular Forces exist in the molecule. Justify your answer. answer. 02 N2 Between the H and the O H2O NH3 Between the H and the N
11. Complete the chart. **You should not have the same answer for all the IMF in the last column** Molecular Formula Lewis Structure(1) Polarity of Molecule Justify your Polarity of Bond. Justify your answer. What type of Intermolecular Forces exist in the molecule. Justify your answer. answer. 02 N2 Between the H and the O H2O NH3 Between the H and the N
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.6QAP
Related questions
Question

Transcribed Image Text:11. Complete the chart.
**You should not have the same answer for all the IMF in the last column**
Polarity of
Molecular
Formula
Lewis
Structure(1)
Polarity of
Molecule
Justify your
answer.
What type of
Intermolecular
Forces exist in
the molecule.
Justify your
Bond.
Justify your
answer.
answer.
02
N2
H2O
Between the H and
the O
NH3
Between the H and
the N
Expert Solution
Step 1
The Lewis structures were given by Lewis who depicts the bonds and electrons present in the molecule.
For O2, the Lewis structure is
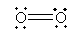
For N2, the Lewis structure is
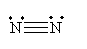
For H2O, the Lewis structure is
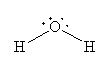
For NH3, the Lewis structure is
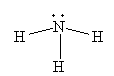
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning