
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
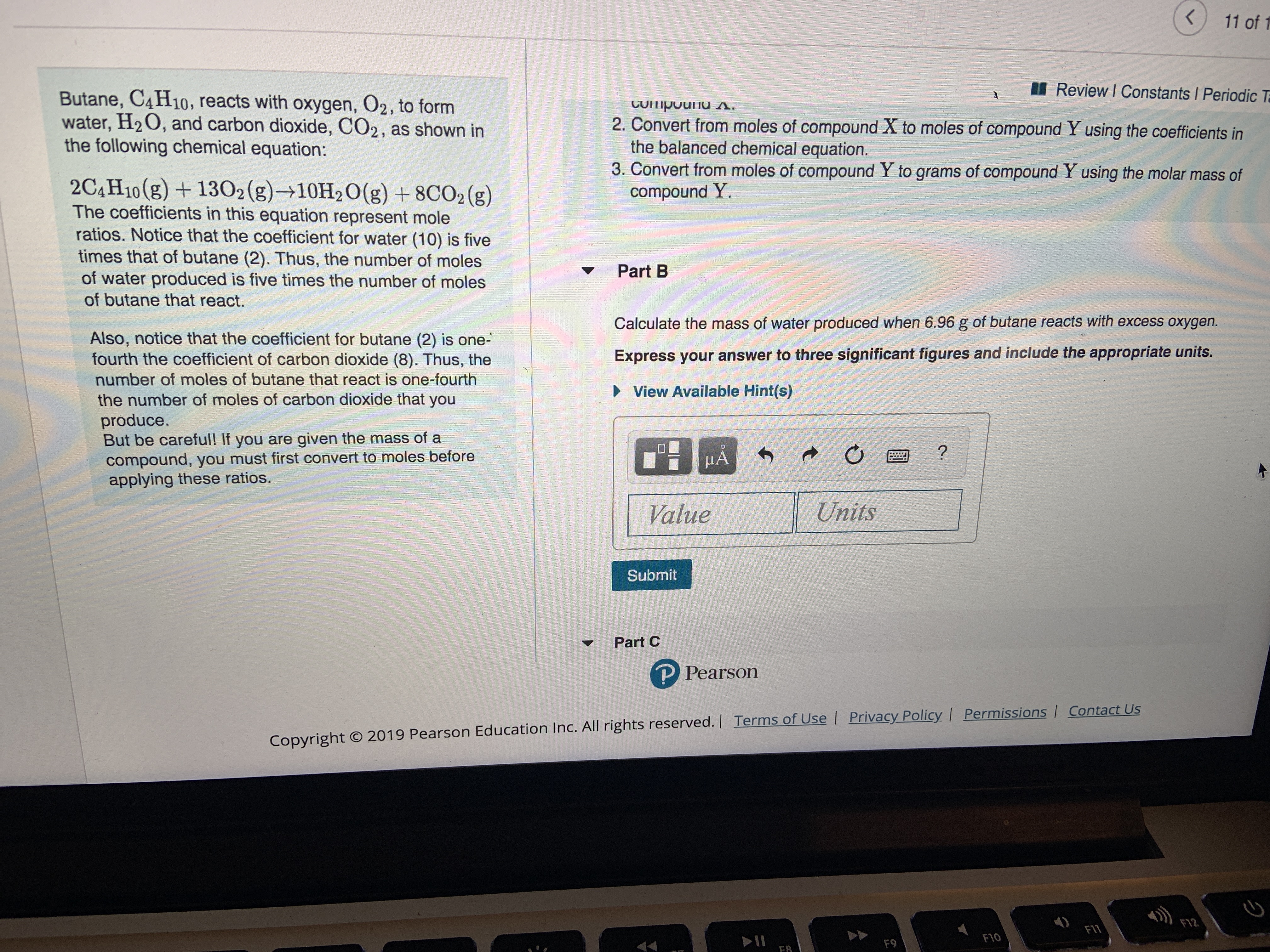
Transcribed Image Text:11 of 1
Review I Constants Periodic T
Butane, C4H10, reacts with oxygen, O2, to form
water, H2O, and carbon dioxide, CO2, as shown in
the following chemical equation:
CUipouu s.
2. Convert from moles of compound X to moles of compound Y using the coefficients in
the balanced chemical equation.
3. Convert from moles of compound Y to grams of compound Y using the molar mass of
compound Y.
2C4H10(g)1302(g)10H2O(g) +8CO2 (g)
The coefficients in this equation represent mole
ratios. Notice that the coefficient for water (10) is five
times that of butane (2). Thus, the number of moles
of water produced is five times the number of moles
Part B
of butane that react.
Calculate the mass of water produced when 6.96 g of butane reacts with excess oxygen.
Also, notice that the coefficient for butane (2) is one-
fourth the coefficient of carbon dioxide (8). Thus, the
Express your answer to three significant figures and include the appropriate units.
number of moles of butane that react is one-fourth
View Available Hint(s)
the number of moles of carbon dioxide that you
produce.
But be careful! If you are given the mass of a
compound, you must first convert to moles before
applying these ratios.
HA
?
Units
Value
Submit
Part C
P Pearson
Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy PolicyI Permissions | Contact Us
F12
FII
F10
F9
F8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Similar questions
- ___________ to ____________ Zn + 2HCl → ZnCl2 + H2 Given Units Unknown Units How many moles of H2 are produced from the reaction of 2.8 moles of HCl?arrow_forwardHow many moles of CO2 are formed by complete reaction of 15.0 moles of octane, C3H18? Calculate Molar Mass of octane, C8H18 Convert 125 pounds of octane to grams, then convert to # moles Calculate the # moles of CO2 released to the environment from combustion of 125 lbs octane above Convert # moles CO2 above to grams, then to poundsarrow_forwardOn a space shuttle, LiOH is used to absorb exhaled CO2 from breathing air to form LiHCO3. How many grams of of LiHCO3 will form if 50.0 g of LiOH used? LiOH(s) + CO2 ⟶ LiHCO3(s)arrow_forward
- Answer the following question using the balanced chemical equation: N₂(g) + 3H₂(g) → 2 NH3(g) How many moles of H₂ are used up if 1.23 moles of NH3 are produced? Your Answer: Answer unitsarrow_forwardHello,please will you help me with the answers. Just need answers not explanation of work.Thank-you! 1.Convert 82.5 g of potassium acetate to moles 2.Convert 9.51 mol of strontium hydrogen carbonate to formula units 3.Convert 3.68 mol AsI3 to molecules 4.Convert 18.6 mol of silver dichromate to formula units 5.Convert 928 g PH3 to moles6.Convert 18.2 mol of silver sulfate to grams7.Convert 14.8 mol of lead (IV) hydrogen phosphate to formula units 8.Convert 8.88 mol of dinitrogen tetrahydride to molecules 9.Convert 5.75 mol Ca to atoms 10.Convert 1.06 x 10^23 formula units of CuSO3 to moles 11.Convert 5.2 x 10^25 formula units of mercury (II) hydrogen sulfate to moles 12.Convert 7.91 x 10^24 formula units of manganese (III) nitrite to moles 13.Convert 647 g Cr2(HPO4)3 to moles 14.Convert 96.6 g KCl to moles15.Convert 610 g of zinc phosphate to moles 16.Convert 98.8 g CoO to moles 17.Convert 18.4 mol NCl3 to molecules 18.Convert 10.8 mol HBr to grams 19.Convert 12.4 mol PbCr2O7 to grams…arrow_forwardAmmonium nitrate (NH4NO3) decomposes to give nitrous oxide (N2O) (laughing gas) and water (H2O): Reaction: NH4NO3 ------- N2O + 2 H2O(l) . How many moles of N2O would be formed if 0.460 moles were decomposed?a.0.92 mol N2O b.1.38 mol N2O c.0.460 mol N2O d.0.613 mol N2O e.1.84 mol N2O How many moles of H2O are formed in the reaction above? a.1.38 mol b.2.76 moles c.5.52 moles d.0.920 moles e.2.30 molesarrow_forward
- An Iron ore sample contains Fe2O3 together with other substances. Reaction of the ore with CO produces iron metal. __Fe2O3(s)+__CO(g)-->__Fe(s)+__CO2(g) a) balance this equation b) Calculate the grams of CO that can react with .350kg of Fe2O3 c) Calculate the grams of Fe and the grams of CO2 formed when .350kg of Fe2O3 reactsarrow_forwardPayalbenarrow_forward17 and 18 thank youarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY