
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
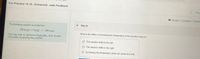
Transcribed Image Text:For Practice 15.16 Enhanced - with Feedback
11 of
I Review | Constants | Periodic
Part A
The following reaction is exothermic:
2SO2(g) + O2(g) = 2SO3(g)-
What is the effect of increasing the temperature of the reaction mixture?
You may want to reference (Pages 665 - 672) Section
15.9 while completing this problem.
O The reaction shifts to the left.
O The reaction shifts to the right.
O Increasing the temperature does not cause any shift.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The elementary reaction 2 H,O(g) = 2 H,(g) + O,(g) proceeds at a certain temperature until the partial pressures of H,O, H, , and O, reach 0.061 bar, 0.0042 bar, and 0.0039 bar, respectively, at equilibrium. What is the value of the equilibrium constant at this temperature? Кр * TOOLS x10arrow_forward2C02 2 2C0+0 Consider the reaction above. If pressure was increase on this equilibrium, how would that affect the amounts of reactants? O They would decrease O They would remain the same O They would increasearrow_forwardPart A It is possible to predict the equilibrium constant of a reaction by combining two or more reactions for which the value of K is known. When combining equilibrium constants, it is important to note the following: Determine the value of the equilibrium constant, Kpal, for the reaction • When a reaction is reversed, its K value is inverted; that is, Kreverse = 1/Kforward- • When the coefficients of a reaction are multiplied by a factor, the K value is raised to the power of that factor. • When reactions are added, their K values are multiplied. CO2 (g) = C(s) + 02(g). Kgoal =? by making use of the following information: 1. 2CO2 (g) + 2H20(1) = CH3COOH(1) + 202(g). K1 = 5.40 x 10-16 2. 2H2(g) + O2(g) = 2H,O(1). K2 = 1.06 x 1010 3. CH3COOH(1) = 2C(s) + 2H2(g) + O2(g). K3 = 2.68 x 10 9 Express your answer numerically. • View Available Hint(s) V ΑΣφ Kgoal = Submit Part B Determine the equilibrium constant, Kroal, for the reaction 4PCI, (g) = P4(s) + 10Cl2 (g), Kgoal =? by making use of…arrow_forward
- 14. The following reaction is allowed to come to equilibrium and then the volume is increased. Predict the effect of the indicated volume change once equilibrium is restored. H2O(g) + CO2(g) double arrow H2CO3(g) The reaction will shift right. The reaction will shift left. There will be no effect. Effect can not be determined from information provided.arrow_forwardCan you provide an explanation? And show the stepsarrow_forwardConsider the equilibrium reaction shown below: 3 NO2 (g) + H2O (l) ⇋⇋ 2 HNO3 (aq) + NO (g) If the total pressure on the system is increased (by compressing the volume), how will the concentration of the NO (g) be affected? Select one: a. The concentration of NO will increase. b. The concentration of NO will decrease. c. There will be no change in the concentration of NO.arrow_forward
- 3. Given the reaction: 2NH3(g) → N2(g) + 3H2(g)Determine the Kc of the reaction at 472°C if the equilibrium concentrations were determined to be[H2] = 0.1270 M[N2] = 0.04020 M[NH3] = 0.002720 Marrow_forwardNetID: Name: 8. What is [NO] and [CO₂] (in M to two decimal places) at equilibrium for the following reaction at 227 °C if the initial concentrations of each reactant is 1.25 M? Explain the validity of your final answer using chemical intuition. NO₂(g) + CO(g) = NO(g) + CO₂(8) -224.09 kJ mol-1 K(227 °C) = 6.9x105 A: [NO] = 1.25; [CO₂] = 1.25arrow_forwardConsider the reaction for the decomposition of hydrogen disulfide: 2H₂S(g) 2H2(g) + S2(g), 1.67 x 10-7 at 800°C K The reaction is carried out at the same temperature with the following initial concentrations: [H₂S] = [H₂] [S₂] 2.00 × 10-4M 0.00 M = 0.00 M Part A Find the equilibrium concentration of S2. Express the concentration to three significant figures and include the appropriate units. [S2] = 1.89 10-5 M Submit Previous Answers Request Answer ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY