
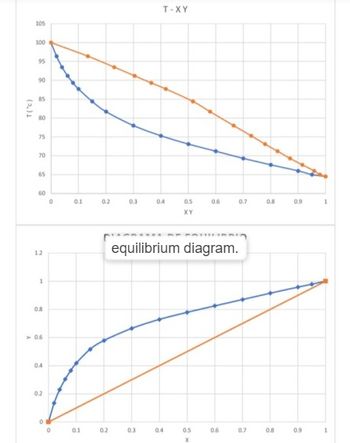
It is desired to separate a mixture of methanol and water in a flash vessel at 1 atm pressure. The equilibrium data are shown in the L-V diagram below. Using this data, answer each of the following questions.
a) The feed has 40 mol% methanol, and V/F = 30%. What are the molar fractions of the vapor and liquid, and the respective flow rates? Assume a feed of 250 kmol/h.
b) If the feed is 35 mol% methanol and it is desired to obtain a liquid product with 15 mol% methanol, what V/F ratio should be used? For a feed of 2000 lbmol/h, find the flow rates and compositions of the products.
c) The tank is operated to obtain a liquid product with 47 mol% methanol. L = 1,800 kmol/h and V/F = 0.2. What are the composition and flow rate of the feed?
d) If z = 0.4 and T = 77°C, find V/F, xA, and yA.
e) If F = 50 mol/h, z = 0.8, and yA = 0.892, find V, L, and xA.
Step by stepSolved in 2 steps

 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





