
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
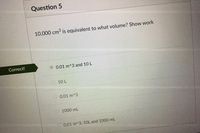
Transcribed Image Text:Question 5
10,000 cm3 is equivalent to what volume? Show work
Correct!
0.01 m^3 and 10 L
10 L
O 0.01 m^3
1000 mL
0.01 m^3, 1OL and 1000 mL
Expert Solution
arrow_forward
Step 1 Given information
Given
Volume = 10000 cm3
To be determined
Volume = ________L
Volume = ________ml
Volume = ________m3
Here we use unit conversion factor , to get the value of volume in specific units.
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [References] Draw structures for the alkene (or alkenes) that gives the following reaction product. Br CH3CH₂CHCCHCH3 II Br CH3 Br₂ • You do not have to consider stereochemistry. • Submit more than one structure only if the structures are constitutional isomers. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the ● Separate structures with + signs from the drop-down menu. e- ChemDoodle {}arrow_forwardThe books answer is Qc = SO2 but I don’t know how it got therearrow_forwardDensity of a Solid (Aluminum) Run I Run II Mass of solid (M) 41.386 g 35.387 g Volume of water 25.32 mL 25.14 mL Volume of water + solid 40.65 mL 38.86 mL Volume of solid (V) mL mL Density of solid (D = M/V) g/mL g/mL Average density g/mL Accepted value for density of solid g/mLarrow_forward
- Chemistry There are 2.55 1015 short tons of oxygen in the atmosphere (1 short ton = 2000 lb). How many metric tons (T) of oxygen are present in the atmosphere (1 metric ton = 1000 kg)? %3D 10 T (Enter your answer in scientific notation.)arrow_forwardTo prepare a solution of KCl, you weigh out 1.5672 (± 0.0003) g and dissolve it in a volumetric flask of 250.00 (±0.50) mL in volume. Express the molarity of the solution and its uncertainty with appropriate number of significant figures. (Formula mass of KCl is 74.551 ± 0.004 g/mol)arrow_forwardYou are given a "Gold Crown" to determine whether it is made of pure Gold or not. It has a mass of 675 grams when it is weighed in the air. Then it is submerged in oil (with density of 957 kg/m^3), it reads 640 grams. 7. Find the density of the Crown. Compare to the Gold density 19320 kg/m^3, is it really pure Gold?arrow_forward
- can you help with putting them in the right orderarrow_forwardIf the volume (V) of a cylinder is calculated as V = is the radius, express the length in terms of V, r, and r. Lar' where L = length and r %3D How many kilometers are equal to 1.58 x 10° feet? If the density of silver is 10.5 /, what is the mass of 1 cubic ft? mL ingarrow_forwardWhen Forrest Gump "just felt like runnin", it is estimated he ran 3.0634 x 10 km. Assuming an average running speed of 6.00 mph and Forrest ran 12 hours a day, how many days was he running? (1milc = 1.60934 km)arrow_forward
- You are in the lab preparing copper solutions for the MICRO lab this week. You measure 0.1035 (±0.0001) g of copper metal and dissolve it in a few mLs of concentrated nitric acid. This solution is diluted to the mark with water in a 50.00 (±0.05) mL volumetric flask. You withdraw a 5.00 (±0.01) mL aliquot with a volumetric pipet and deliver it to a 100.00 (±0.08) mL volumetric flask. After dilution to the mark with water, calculate the concentration and the absolute error in a solution of copper in ppm (assume ppm = 1 µg/g = 1 µg/mL = 1 mg/L).arrow_forwardQUESTION 4 Measurements & Density In this experiment, students are asked to determine the density of an unknown solid. A student enters the lab and obtains a cubic object of unknown material. The mass of the object is found to be 18.9325 g. When measuring the length, this is what the student sees: cm 11 2 3 41 5 6arrow_forwardhow to convert 4507 kg m^3 into g/mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY