
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Question is attached please explain step by step thank you
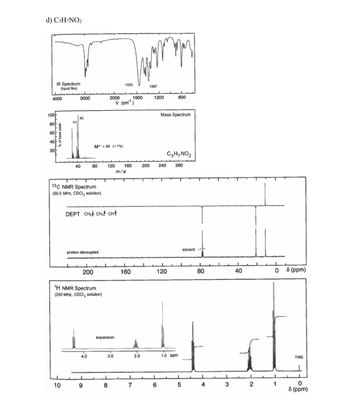
Transcribed Image Text:100
80
60
20
d) C3H7NO2
IR Spectrum
1553
1387
(liquid film)
سلا
4000
3000
2000
1600
1200
800
V (cm³)
% of base peak
411
43
M+*=89 (<1%)
Mass Spectrum
C3H7NO2
40
80
120 160
m/e
200
240
280
13C NMR Spectrum
(50.0 MHz, CDCI, solution)
DEPT CH₂ CH, CH↑
proton decoupled
solvent
L
L
200
160
120
80
40
0
8 (ppm)
1H NMR Spectrum
(200 MHz, CDCI, solution)
T
expansion
4.0
3.0
2.0
1.0 ppm
10
9
8
7
6
5
4
3
2
1
TMS
0
$ (ppm)
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- 4000 IR Spectrum quid film) 100 80 60 40 YI 2248 41 10 3000 43 40 proton decoupled "C NMR Spectrum (150 MHz CDC, solution) 68 DEPT CH, CH, 1 CHT 9 200 ¹H NMR Spectrum (600 MHz, CDC, solution) M83 (<1%) 80 120 2000 v (cm) 8 m/e 160 160 2.3 L 7 1600 2.2 200 ryper 1200 6 ▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬ 120 Mass Spectrum 240 21 2.0 expansions (not to scale) 800 I CsH₂N 280 5 expansion 80 4 270 255 256 255 expansion No significant UV absorption above 220 nm 27.0 245 250 255 1.0 3 40 L 2 0 8 (ppm) 1 1 TMS L 1 0 8 (ppm) 10 ¹H-¹C me-HSQC Spectrum (600 MHz, CDC, solution) Black cross-peaks CH and CH Red cross-peaks CH₂ ppm 20 ¹H-"C HMBC Spectrum (600 MHz, CDC), solution) -22 -24 -26 -28 116 118 120 122 124 3.0 2.8 2.6 2.4 2.2 2.0 1.8 1.6 1.4 1.2 1.0 0.8 0.6 ppm ppm 20 22 -24 26 28 -116 118 -120 122 -124 3.0 2.8 2.6 2.4 2.2 2.0 1.8 1.6 1.4 1.2 1.0 0.8 0.6 ppm IR: List bands and possible/probable structural units responsible Band (cm³) Possible…arrow_forward4000 100 80 60 20 % of base peak 3376 40 10 3000 59 13C NMR Spectrum (50.0 MHz, CDCI, solution) 173 proton decoupled 200 I 9 80 ¹H NMR Spectrum (200 MHz, CDCI, solution) DEPT CH₂ CH₂ CH4 IR Spectrum (liquid film) 2000 M+88 (<1%) 120 1600 v (cm¹) m/e 160 160 I I 8 7 mmm 1 120 200 240 6 1200 . Mass Spectrum L 800 1 5 C5H₁20 280 solvent L 80 4 problem 78 3 No significant UV absorption above 220 nm 40 Il 08 (ppm) 1 2 1 TMS 0 8 (ppm)arrow_forwardIR Spectrum (liquid film) 184 4000 100 80 60 40 20 40 13C NMR Spectrum (50.0 MHz, CDCI, solution) proton decoupled 200 ¹H NMR Spectrum (600 MHz, CDCI, solution) 1 10 9 8 3000 % of base peak 80 128 my 2000 V (cm¹) 119 M+* 134 120 160 m/e expansion 124 ppm 160 1600 I 7 200 I 6 120 ppm 1200 800 Mass Spectrum C10 H14 280 solvent 80 240 I 5 7.4 1 4 expansion 3 Problem 95 UV Spectrum max 262 nm (log10 € 2.5) solvent: ethanol -CH3 C 40 7.2 2 1 0 8 (ppm) TMS 1 I 1 0 8 (ppm) 201arrow_forward
- Myriam 1718 1. 4000 3000 1600 1200 800 V (cm¹) 100 Mass Spectrum 43 119 80 60 JIK M+* 40 176 20 161 C12 H16 O 40 80 200 280 120 160 m/e 13C NMR Spectrum (50.0 MHz, CDCI, solution) expansion 188 IR Spectrum (liquid film) % of base peak DEPT CH₂ CH₂ CH proton decoupled L 200 ¹H NMR Spectrum (200 MHz, CDCI, solution) J 1 9 10 I 8 2000 160 the 1 7 6 240 120 130 130 125 ppm 125 ppm L 5 expansion 1 80 1 4 solvent Problem 99 UV Spectrum max 262 nm (log₁0 € 2.5) solvent methanol 40 t I 3 1 2 4 08 (ppm) TMS 1 0 8 (ppm) 1 1 205arrow_forwardWhat is the IR and HNMRAndCNMR and mass in this examplearrow_forwardWhat is the IR and HNMRAndCNMR and massarrow_forward
- 13C NMR Analysis of Aspirin Chemical Shift (Observed ppm) Assignment(s) (A'-J') Expected Chemical Shift Range (ppm) B' D' E' JOH Acetylsalicylic Acid (Aspirin) 169 & 170 152 125 135 (4 peaks) - 122 20 160 140 120 100 Figure 14.13 125.7-MHz 13C-NMR spectrum of aspirin in CDCl3. solvent 80 60 40 20 ppmarrow_forwardinterpret/identify the peaks and their characteristics. (ex. C=O, 4H, doublet) compound name: isopentyl acetate (C7H14O2)arrow_forwardIR Spectrum (liquid film) لللللللللللا 4000 100 80 60 408 20 40 3000 39 10 proton decoupled "C NMR Spectrum (100 MHz, CDC, solution) 75 200 9 ¹H NMR Spectrum (400 MHz, CDCI, solution) 77 5.50 558 expansion 188- ▬▬▬▬▬▬ 2000 T 8 M+ 110/112/114 80 120 160 200 240 m/e V (cm) DEPT CH₂ CH₂ 1 CH 1 160 1600 5.42 5.40 ppm mp 7 L W 6 1200 120 Mass Spectrum 800 5 C3H4Cl2 280 solvent T I 80 1 4 3 No significant UV absorption above 220 nm 40 expansion 4.18 4.16 pom 111 2 08 (ppm) 1 1 TMS L 0 ō (ppm) IR: List bands and possible/probable structural units responsible Band (cm³) Possible stretches/functional groups MS: Molecular Formula 13C NMR: 1 2 3 4 5 6 Chem. Shift (8) Type of C (sp, sp², carbonyl, etc.) ¹H NMR: 1 2 3 4 5 Chem. Shift (8) Multiplicity (singlet, doublet, etc.) IHD(DU)= (Show calculation) 6 If you need more space, add any additional signal info here: # of attached H's #of identical C's in signal Probable Type of H (methyl, aromatic, etc.) # of identical H's in signalarrow_forward
- Unrounded Rounded ε∗,L/μmol 0.0259825 0.0260 Heres the data if needed: TZ # Concentration Absorbance at 430 nmnm 1 33.6480 0.931 2 25.2360 0.757 3 16.8420 0.210 4 8.41200 0.137 5 4.20600 0.122arrow_forwardDeduce what is the unkown compounds. Please label the peaksarrow_forwardPlease don't provide handwriting solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY