
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
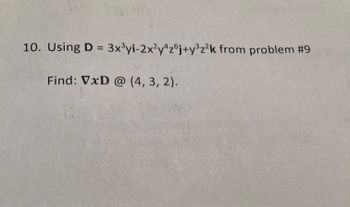
Transcribed Image Text:Dec Daistby
10. Using D = 3x³yi-2x²y4z6j+y³z²k from problem #9
Find: VxD @ (4, 3, 2).
Dal
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- = = Imagine that we have a box that emits electrons in a definite but unknown spin state y). If we send electrons from this box through an SGz device, we find that 20% are determined to have Sz +ħ and 80% to have S₂ -ħ. If we send electrons from this box through an SGx device, we find that 90% are determined to have Sx +ħ and 10% to have Sx Determine the state vector for electrons emerging from the box. You may assume that the vector components are real. -1/ħ. = -arrow_forwardCan you explain how we get 8.25 from substituting 8.24 in 8.23?arrow_forwardUse the Energy equation to Calculate E1, E2, E3, E4, E5, and E6 ( in joules) Balmer Series; a. Calculate the transition frequencies (Hz) in the Balmer series f1 = (E6- E2)/ h, f2 = (E5 - E2)/h, f3 = (E4- E2)/ h, f4 = (E3 - E2)/h. b. Use the internet to get the type of optical wave corresponding to each frequency. Lyman Series; a. Calculate the transition frequencies (Hz) in the Lyman series f1 = (E6- E1)/ h, f2 = (E5 - E1)/h, f3 = (E4- E1)/ h, f4 = (E3 - E1)/h, f5 = (E3 - E1)/h. b. Use the internet to get the type of optical wave corresponding to each frequency.arrow_forward
- What is the magnitude of the electric field at a distance of 0.1 nm from a thorium nucleus? I have tried 2.304E-8 and 2.07E-6 and they are both wrongarrow_forwardProblem 7: Consider what you've learned so far regarding the nucleus of an atom.arrow_forwardQuestion 7 The wave function of an electron in a hydrogen - like atom is (r) = Cea where a = ao/Z with a being the Bohr radius and Z the atomic number. The nuclear charge is Ze and the atom contains only one electron. a) Compute the normalization constant. b) If the nuclear numbers are A = 173 and Z= 70, what is the probability that the electron is in the nucleus of radius R? Assume R = 1.2x4¹/³ fm 0.arrow_forward
- = Using the formula for the hydrogen atom energy levels, En constant can be written in terms of fundamental quantities, RH = Me 4 8€ ²h³c Me4 1 860²h² n²¹ the Rydberg and its value approaches, RH → R = 10,973,731.6 m¹ in the limit μ→ me. (a) How would this constant be defined for a one-electron species containing Z protons in its nucleus? Consider how this changes the form of the Hamiltonian and the energy levels for that Hamiltonian. (b) The hydrogen atom emission lines in the Balmer series (n₂ = 2) lie in the visible portion of the electromagnetic spectrum. Would this also be true if Z> 1? Find the wavelength (in nm) of the n = 32 emission in hydrogen and that for a one-electron species with Z = 2. (You will be asked to report a quantity on the quiz that depends on these two values.)arrow_forwardExplain the physics behind the fourth assumption in Bohr's atomic model, i.e., mevr-nh/2, where me and v are the mass and velocity of electron around the nucleus, respectively, r is the radius of an allowed orbit, n is an integer and h is the Plank constant.arrow_forwardWhat are (a) the x component, (b) the y component, and (c) the z component of ?→=?→−?→+?→�→=�→-�→+�→ if ?→=7.1?̂ +5.0?̂−2.1?̂�→=7.1�̂+5.0�̂-2.1�̂ , ?→=−8.9?̂ +8.6?̂+2.8?̂�→=-8.9�̂+8.6�̂+2.8�̂ , and ?→=1.1?̂ +4.1?̂+2.5?̂�→=1.1�̂+4.1�̂+2.5�̂ . (d) Calculate the angle between ?→�→ and the positive z axis. (e) What is the component of ?→�→ along the direction of ?→�→ ? (f) What is the magnitude of the component of ?→�→ perpendicular to the direction of ?→�→ but in the plane of ?→�→ and ?→�→ ?arrow_forward
- Why does this part not require a Sin(30) to decompose the MG(Sin(30))(.8)? The example gives a correct answer.arrow_forwardIs this the correct way to solve for W(ca)arrow_forwarda. Is a 4p → 4s transition allowed in sodium? If so, what is its wavelength? If not, why not?b. Is a 3d → 4s transition allowed in sodium? If so, what is its wavelength? If not, why not?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON