
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please answer all parts , thank you :)
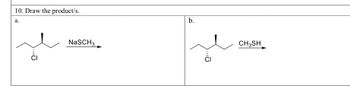
Transcribed Image Text:**Question 10: Draw the product/s.**
**a.**
The first reaction involves a chlorinated compound reacting with sodium methanethiolate (NaSCH₃). The compound has a chiral center with chlorine as a leaving group.
**b.**
The second reaction involves the same chlorinated compound reacting with methanethiol (CH₃SH). In both reactions, consider possible nucleophilic substitution mechanisms and stereochemistry changes.
(Note: Actual product structures are not provided in the image and would need to be drawn based on chemical knowledge of the reactions.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I IUIAC-ION340DC564eecac03101af2döf56bb#10301 Home-AP Student. Elk Grove Unified - O My Profile - Zoom Results of the dhec.. VMy Citation list 9/2.. O Pearson Sign In 6 College Board -SAT. Reading list You may want to reference (Pages 258 - 262) section 6.4 while completing this problem. Constants Perodic Table Part A An unknown mass of each of the following substances, initially at 25.0 °C. absorbs 1960 J of heat. The final temperature is recorded as indicated. Find the mass of each substance. a. Pyrex glass (T = 55.6 C) Express your answer using two significant figures. m = Submit Previous Answers Request Answer X Incorrect; Try Again; 14 attempts remaining Part B sand (Ty = 62.3 C) P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy Permissions | Contact Us | V I 2:42arrow_forwardAutoSave w | homework – Saved to my Mac OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe Paste BIU V ab x, x A v I v A v No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity Please answer the following questions fully and to the best of your ability 1) Please state how you would synthesize the following polymer below and show the mechanism behind the reaction. Note that this also means you need to tell me which molecule you are starting with as well as specifying all conditions required. он OH 2) Will either the polymer or the monomers you used to make it show peaks if IR spectroscopy analysis was done on them, and if so where? How about if UV-vis spectroscopy was performed instead? Page 1 of 1 93 words E English (United States) O Focus 白arrow_forwardas X Clas X ||| STE X © Ban X Jen Scho × Ban X CD www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNsikr7j8P3jH-liG_IZvpRqwiHv-fgOzocXR7H3QULJsrn-hH7iM_OKtS081EM1kmGkq29c dent Bookmarks 00 Duolingo - The worl... ▸ YouTube Mary G. Ross: Who... Zomberry Hero > Try Again Cha X O MATTER Finding the side length of a cube from its volume in liters Your answer is incorrect. 0.75 m Trial X Explanation A technical machinist is asked to build a cubical steel tank that will hold 475 L of water. Recheck 198 X Calculate in meters the smallest possible inside length of the tank. Round your answer to the nearest 0.01 m. → # X $ " % d H 6 H Copy of Cause and... FORENSIC SCIENC... kh hp Bay x M & 7 Ⓒ2023 McGraw Hill LLC. All Rights Re C Cop X * 00 8 ( 9arrow_forward
- hrome File Edit View History Bookmarks Profiles Tab Window Help 令Q 2 O Mon Apr 25 Watch Gilmore Girls | Ne x a ALEKS A ALEKS - Reyna Garcia A ALEKS - David Teague 9,511 Jobs in Wilmington x O New Tab A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-lvdWKW BBZZ16tTytly4Fcfu6zOtOf8oMM9sQvs3exlZv5th3sCgLJcZoF3sZYH-. O R Paused O Spotify Web Playe.. M Common Ethical D.. 国 Objective Knowledge Check Question 11 Reyna V Aqueous hydrochloric acid (HCl) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H,O). Suppose 4.37 g of hydrochloric acid is mixed with 7.0 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. 圖 dlo IIarrow_forwardThe inital points are ( 0, 27.12) and (0.00250 , 5.17)arrow_forwardAutoSave We hw OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo.. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe BIU V ab A • I v A v Paste х, х E= == No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity C13 NMR Peaks Aldehydes RCO)R Aldehydes and ketones Carbaxylic R(CO)X Carbaxylic acid derivatives Ntrile Nitrile RCN CC C-C Alkyne Akyne R-CC-R RCH20 RCH2-O R4C RAC R3CH R3CH RCH2X X= C-C, C-O, Br, CI, N RCH2X R2CH2 RECH2 RCH3 RCH3 TMS TMS 220 200 180 160 140 120 100 80 60 40 20 Typical chemical shifts in 13c-NMR 9) Why do the peaks of associated with aldehydes (both in H NMR and C NMR) appear so much further downfield than other peaks? C13 NMR Peaks E Page 5 of 5 E English (United States) O Focus 295 words 白arrow_forward
- 3.14 Show that Tx = 1+ T(0 In Z/0T),, and that px = 1 - p(0 In Z/ap).arrow_forwardLearning AA prod03-cnow-owl.cengagenow.com Login Learning Learning × Online tea... y dr. marlow... ember L... with We 3. AS surroundi... M 2BrF3 (9) Br2 (g) + 3F2 (9) 4. AG° = AH°... M 5. AG: Pre... 1req 6. AG: Enthal... Using standard thermodynamic data at 298 K, calculate the free energy change when 2.14 moles of BrF3 (9) react at standard conditions. Substance AG (kJ/mol) 7. AG fro... 1req Question Question Question 8. Calculat... 1req AG° = 9. Calculat... 1req BrF3 (9) -229.4 Br2(g) 3.1 F2(9) 0.0 kjarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY