
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
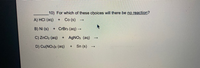
Transcribed Image Text:10) For which of these choices will there be no reaction?
A) HCI (aq)
Co (s)
+
B) Ni (s)
+ CrBr3 (aq) -
C) ZnCl2 (aq)
AGNO3 (aq)
D) Cu(NO3)2 (aq)
Sn (s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the Net Ionic equation for this reaction.arrow_forwardWhat is the balanced net ionic equation for this reaction. Pb(NO,),(aq) + 2HCI(aq) PBCI, (s) + 2HN0,(aq) HCl and HNO, are both strong acids.arrow_forwardExp # 2: suppose you have a {Zn2+(aq), Fe3+(aq)} solution, and you’re asked to separate the two ions, how do you seperate the ions and form a precipitate?arrow_forward
- Give detailed Solution with explanation needed..don't give Handwritten answer...don't use Ai for answering thisarrow_forwardWrite net ionic equations for the following reactions:arrow_forward13 Balance these net ionic equations. (a) Ag+(aq) + Br¯(aq) → AgBr(s) (b) Cd2+(aq) + S?-(aq) → CdS(s) (c) Sc³+(aq) + SO̟²-(aq) (d) Sn²+(aq) + Fe2+(aq) → Sn(s) + Fe3+(aq) (e) K(s) + H,O(E) –→ K*(aq) + OH¯(aq) + H,(g) → Sc,(SO,)3(s) 4arrow_forward
- Write the net ionic equation for the following molecular equation. MnBr2 (aq) + (NH,)2CO3 (aq) → MnCO3 (8) + 2NH,Br(aq) (Use the lowest possible coefficients. Be sure to specify states such as (aqg) or (s). If a box is not needed, leave it blank.)arrow_forwardA sample of a chloride salt weighing 484.6 mg is titrated with AgNO3 using K2CrO4 as indicator. To reach the end point a 52.3 mL of 0.1475 M of AgNO3 solution is required. The net ionic equation for the reaction of titration is: Ag+ (aq) + Cℓ- (aq) → AgCℓ (s) How many moles of Ag+ (aq) ions are used to reach the end point? How many moles of Cℓ- (aq) have reacted in the titration? How many grams of chloride are present in the sample? What is the mass percent of chloride in the sample?arrow_forwardGive the net ionic equation for this unbalanced reaction: CaCl2 (aq) + AgNO3 (aq) →Ca(NO3)2 + AgCl (s)arrow_forward
- Why is this equation not a redox reaction? Fe2(SO4)3(aq) + 3H2O(l)arrow_forwardUse the activity series to predict which of the following reactions will occur. Complete and balance the equations. If no reaction occurs, write “no reaction” as the product. (a) Cu(s) + NiCl2(aq) ------> (b) Rb(s) + H2O(l) ------> (c) I2(s) + CaCl2(aq) ------>arrow_forward2 MnO4−(aq)+5 H2C2O4(aq)+6 H+(aq)→2 Mn2+(aq)+10 CO2(g)+8 H2O(l) The reaction between MnO4−(aq) and H2C2O4(aq) in acidic solution is represented above. In a titration experiment, a 0.040 M solution of KMnO4(aq) is added from a buret to an acidified sample of H2C2O4(aq) in a flask. The endpoint of the titration is determined by the appearance of a faint pink color in the mixture. Initial buret reading 6.09 mL Final buret reading 26.09 mL Based on the data in the table above, how many moles of H2C2O4(aq) were in the sample?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY