
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
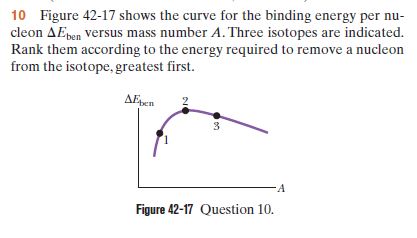
Transcribed Image Text:10 Figure 42-17 shows the curve for the binding energy per nu-
cleon AEpen versus mass number A. Three isotopes are indicated.
Rank them according to the energy required to remove a nucleon
from the isotope, greatest first.
AEpen
-A
Figure 42-17 Question 10.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Calculate the binding energy per nucleon for 232Th, ¹2C, 78Se, and 5⁹ Co. (For the atomic masses, see this table. Enter your answers to at least two decimal places.) (a) 232Th (b) 12C (c) (d) 78 Se 59 CO MeV/nucleon MeV/nucleon MeV/nucleon MeV/nucleonarrow_forwardPlease make sure you are sufficiently precise in your answers that the BE per nucleon can be calculated to 1% 08Zn has atomic mass 67.9248476 u. What is the mass in MeV/c2? Number What is the binding energy in MeV? Number What is the binding energy per nucleon in MeV? Number Estimate the radius of this nucleus. Give your answer with units. Number Unitsarrow_forwardCalculate the binding energy for the following nuclides; expressd the binding energy in: (i) J/nucleon, and (ii) MeV/nucleon. (a) s (atomic mass = 31.97207 u) (b) 2U (atomic mass = 235.04393 u) (Atomic masses: proton (H = 1.00728 u; neutron (¿n) = 1.00867 u; electron (-je) = 0.00055 u; 1 u = 1.6605 x 10-2" kg; speed of light, c = 3.00 x 10 m/s; 1 MeV = 1.602 x 10-13 J) 7.arrow_forward
- Two nuclei having the same mass number are known as isobars. (a) Calculate the difference in binding energy per nucleon for the isobars 1123Na and 1223 Mg. (b) How do you account for this difference? (The mass of 1223Mg =22.994 127u)arrow_forwardIf an atoms with 62 protons undergoes β-decay, the daughter nuclide will have ____ protons.\arrow_forwardCalculate the binding energy per nucleon for the boron-10 nucleus, whose mass is 10.012937 u.The binding energy per nucleon:arrow_forward
- Calculate the binding energy per mole of nucleons for sodium-23. Masses needed for this calculation are (in g/mol) H = 1.00783, n = 1.00867, and Na = 22.98977. (Na) = n kJ/mol nucleonsarrow_forwardA plot of binding energy per nucleon versus the mass number (A) shows that nuclei with a small mass number have А a small binding energy per nucleon, as the mass number increases the binding energy per nucleon increases, and the value for the binding energy per nucleon has a maximum value for nuclei with a mass number around 60. Verify that this is the case by determining the binding energy per nucleon for each of the following four nuclei. (Let the mass of a proton be 1.0078 u, the mass of a neutron be 1.0087 u, the mass of 'He be 3.0160 u, the mass of Li be 8.0225 u, the mass of 2Ni be 61.9283 u, and the mass of 112, Cd be 111.9028 u. Enter your answers in MeV and to at least three significant figures.) (а) ЗНе Review the meaning of the A, Z and N numbers. Review how to find the binding energy of a nucleus and then the binding energy per nucleon. MeV (b) 8Li MeV (c) 62NI MeV (d) 112cd MeVarrow_forwardDetermine the energy released when a Uranium-235 nucleus under fission to Te-134, Zr-99, and two neurons. A table of isotopes lists the binding energy per nucleon on the nuclei of U-235, Te-134, and Zr-99 as 7.69 MeV, 8.38MeV, and 8.54MeV, respectively.arrow_forward
- In the figure below, note that for low Z, all the nucleii with the most highest binding energy per nucleon have both an even number of protons and an even number of neutrons. Explain why this is. 9 soFe 9%Mo 160 8. 144N. 183W He 20sPb "Be 235U 6. Fusion reactions release energy Fission reactions release energy 1 40 80 120 160 200 240 Atomic Mass Number (A) Use the editor to format your answer E (MeV)arrow_forwardFind the binding energy and binding energy per nucleon of the 27/13 Al nucleus.arrow_forwardif you have two vectors : A=-2i+3j-k B=-i+3j-k find : 1. -3B 2. 2A-3B 3. Direction of A 4. Direction of Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON