
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
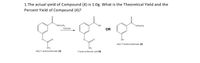
Transcribed Image Text:1.The actual yield of Compound (4) is 1.0g. What is the Theoretical Yield and the
Percent Yield of Compound (4)?
rOCH CH3
OCH,CH3
Esterase
OR
OH
ethyl 3-hydroxybenzoate (2)
ethyl 3-acetoxybenzoate (3)
3-acetoxybenzoic acid (4)

Transcribed Image Text:ÓH
(1)
Ac20 / H3PO4 / 75°C
ČH3
(4)
Scheme 2:
Acylation of (1)
To a stirred solution of hydroxybenzoic acid 1 (1.0 g, 7.2 mmol) and acetic anhydride (2.5 mL,
26.4 mmol, 3.7 equivalents) was added 3 drops of concentrated phosphoric acid. The
solution was stirred and warmed to 75°C on a water bath until TLC (1:2 EtOAc:Heptane)
deemed the reaction complete. Afterwards, the reaction mixture was removed from the
water bath, 15 mL of water was added and the precipitate that formed was vacuum filtered.
The resulting solid was dissolved in a conical flask in 3 ml of hot ethanol and then poured
into 15 ml of water. After cooling the solution in an ice bath, the resulting precipitate was
vacuum filtered and left as such for 15 min to dry the crystals.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1 Complete the following table by filing in the boses with the appropriate starting material, rengent or product 1. NANH, NH, 2 EIBr LUndars catalyst 1. BH-THF 2. NICH, HO NaGH DMSO 2 equivalence HBrarrow_forwardKk.9.arrow_forward2) Which reactant is oxidized in the following reaction: 12 + 2S2032- = 21 + S40g²-arrow_forward
- Which of these compounds is most reduced? a) C2H6 (ethane) b) CH3CH2OH (ethanol) c) CH3CHO (acetaldehyde d) CH3COOH (acetic acid)arrow_forwardDraw the product of the reactionarrow_forwardWhich reagent has been oxidized in the following reaction: H2(g) + N2(g) --> NH3(g) 1- H2 2 - N2 3 - NH3 4 - all have been reducedarrow_forward
- Give the major product(s) of the following reaction. CH3CH2CH2CH2CI (1 mole) AICI3, heatarrow_forward7. What is the theoretical yield of the ester from the reaction of 11.6 grams hexanoic acid with 7.4 grams of 2-methyl-1-propanol? H2 HO, H3C H2 Chemical Formula: C,H1202 Molecular Weight: 116.16arrow_forwardPredict the products of the following biochemical reaction: السلام CH₂ CH CH₂2 + 3 H₂O H* A P In particular, draw the structure of the product or products P in the drawing area below. If there are no products, because this reaction won't happen, check the No reaction box under the drawing area. Note: if there is more than one product, you can draw them in any arrangement you like. Also, just draw the structure of each product. You don't have to draw the complete right-hand side of the equation, including stoichiometric coefficients. Click and drag to start drawing a structure. X :0arrow_forward
- 16 In the body, during glycolysis the following conversion occurs in a 2-step process. 0₂PO. OH OK₂Cr₂O7 NaBH4 ہلہ H₂SO4 and heat LiAlH4 H₂O 0₂ PO. OH What reagent(s) would you use to bring about this conversion in the laboratory? OHarrow_forwardDraw the intermediate product and the final product for the synthesis of ethyl 3-coumarin carboxylate.arrow_forwardDon't use AI.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY