
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Gg.139.
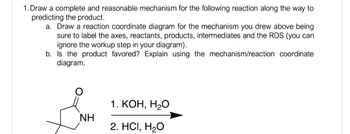
Transcribed Image Text:1. Draw a complete and reasonable mechanism for the following reaction along the way to
predicting the product.
a. Draw a reaction coordinate diagram for the mechanism you drew above being
sure to label the axes, reactants, products, intermediates and the RDS (you can
ignore the workup step in your diagram).
b. Is the product favored? Explain using the mechanism/reaction coordinate
diagram.
NH
1. KOH, H2O
2. HCI, H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the following equations using the smallest possible whole number stoichiometric coefficients. Do not include the states of matter. Part 1 of 3 Ba + 0, -BaO Part 2 of 3 H₂O₂ H₂O + 0₂ 0-0 X 00 ローロ X 3 00 09 5 Save For Later ○ 民国图] E allo Ar Submit Assignmentarrow_forwardWrite out the nuclear energy configuration (energy configuration of protons and neutrons in the nucleus, nuclear orbitals) of Calcium-40 and use it to explain why Ca-40 is an exceptionally stable and the most common isotope of Ca.arrow_forwardF с Br + K ОН Br Н OCH 3 CI M я ОН С NHarrow_forward
- B CH 3 11 Br 11117 CH 3 онarrow_forwardBr Li Et₂0 Cul I CHCI3 (CH3)3CO-Karrow_forwardK²=32 2016 #H HE M = PENNS 00.5 = [14] (MOOS = [V] [MOLO FAVOROins nos 40241 23 enorpos 09 10 1011 3 MUNGHOUD? 1D 21 NT A 00091 907 559100 21 no inw 1/09 2000 200 DAD91 SNT 8 SNT.S (s) + 6C1₂ (9) = 4P(|3 (9) P₁ Express the equalibrium constant for this reactionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY