
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the equation
3H2(g)+N2(g)→2NH3(g)3H2(g)+N2(g)→2NH3(g)
The ammonia produced in the Haber-Bosch process has a wide range of uses, from fertilizer to pharmaceuticals. However, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation.
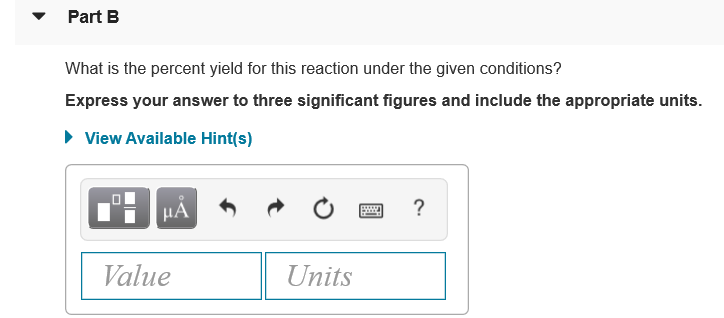
Transcribed Image Text:• Part B
What is the percent yield for this reaction under the given conditions?
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
μΑ
Value
Units
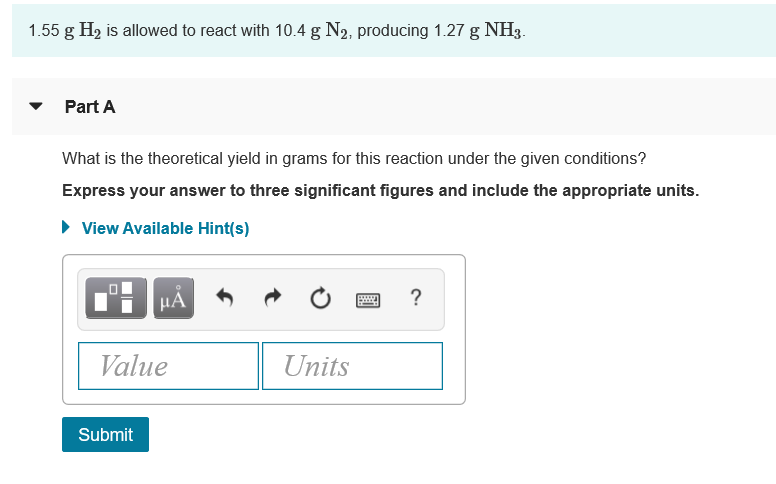
Transcribed Image Text:1.55 g H2 is allowed to react with 10.4 g N2, producing 1.27 g NH3.
Part A
What is the theoretical yield in grams for this reaction under the given conditions?
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
HÀ
Value
Units
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15. Kkura found a sulfide ore in the dungeon which she identified to be chalcocite. While waiting for her debut, she read from a journal that grinding and "roasting" (heated strongly with oxygen gas) chalcocite can generate copper (1) oxide, a pigment used to color ceramics. Find the amount of copper(I) oxide she can produce from 53 grams copper(I) sulfide. Cu2S(s) + O2(g) –> Cu2O(s) + SO2(g)arrow_forwardThe chemical equations below show reactions similar to those you have met already. One of them is balanced correctly, and three are not. Identify which one is correctly balanced. State why. - 4 Fe (s) + 3 O2 (g) = 2 Fe2O3 (s) - Mg (s) + O2 (g) = 2 MgO (s) - 2 K (s) + 2 O2 (g) = 2 K2O (s) - 2 Na (s) + H2O (l) = 2 NaOH + H2 (g)arrow_forwardThe reaction between ammonia and oxygen is given below: 2 NH3(g) +2 O2(g) → N2O(g) +3 H₂O(1) We therefore know that which of the following reactions can also occur? N2O(g) +3 H2O(l) → 2 NH3(g) +2 O2(g) 4 NH3(g) +5 O2(g) →4 NO(g) + 6 H₂O(g) 4 NO(g) +6 H2O(g) →4 NH3(g) +5 O2(g) None of the Abovearrow_forward
- Consider the synthesis of ammonia: N2 (g) +3H, (g) 2NH3 (g)arrow_forwardThe extraction of aluminum metal from the aluminum hydroxide found in bauxite by the Hall-Héroult process is one of the most remarkable success stories of 19th century chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina Al2O3 and water: 2Al(OH)3(s) + Al2O3(s) -> 3H2O(g) In the second step, alumina Al2O3 and carbon react to form aluminum and carbon dioxide: 2Al2O3(s) + 3C(s) + 4Al(s) -> 3CO2(g) Suppose the yield of the first step is 74.% and the yield of the second step is 76.% . Calculate the mass of aluminum hydroxide required to make 4.0kg of aluminum. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits.arrow_forward1arrow_forward
- Suppose a pair of chemical compounds A and B can react in two different ways: A+B C Reaction 1 gives product C. A+B D →> Reaction 2 gives product D. The following facts are known about the two reactions: • Reaction 1 is endothermic and Reaction 2 is exothermic. • If a reaction vessel is charged ("filled") with A and B, then at first C is produced faster than D. Use these facts to sketch a qualitative reaction energy diagram for both reactions. Note: because these sketches are only qualitative, the energies don't have to be exact. They only have to have the right relationship to each other. For if one energy is less than another, that fact should be clear in your sketch. energy A + B Reaction 1 reaction coordinate energy A + B Reaction 2 reaction coordinate ☑arrow_forwardWhich reaction has the largest AS? ON₂(g) + 3H₂(g) → 2NH3(9) □2NO(g) → N₂O2(g) O2N₂ H₁(g) →→→ 2NH3(g) + H₂(g) □ O₂(g) + 2H₂(g) → 2H₂O(g) □ O2(g) +2H₂(g) → 2H₂O(l)arrow_forwardBlast furnaces extract pure iron from the iron(III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide: 2C(s)+O2(g)→2CO(g) In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide: Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g) Write the net chemical equation for the production of iron from carbon, oxygen and iron(III) oxide. Be sure your equation is balanced.arrow_forward
- Balance the following chemical equation (if necessary): C₃H₆(g)+O₂(g)→CO₂(g)+H₂O(g)arrow_forwardConsider the following reactions:CoO (s) + CO (g) D CO2 (g) + Co (s) Kc(1) = 490.2 CoO (s) + 2 H2 (g) D 2 Co (s) + 2 H2O (g) Kc(2) = 4.5 x 103a. Write the overall equation for the reaction of hydrogen gas and carbon dioxide gas to produce carbon monoxide gas and steam.arrow_forwardWhat is true for this chemical reaction: CO₂(g) + 4H₂(g) = CH₂(g) + 2H₂O(g)? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b C Reaction Changes Homework Unanswered Due Today, 11:59 PM d AHrxn > 0; ASrxn > 0 AHxn 0 AHxn 0; ASxn < 0 Unanswered. 2 attempts leftarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY