
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

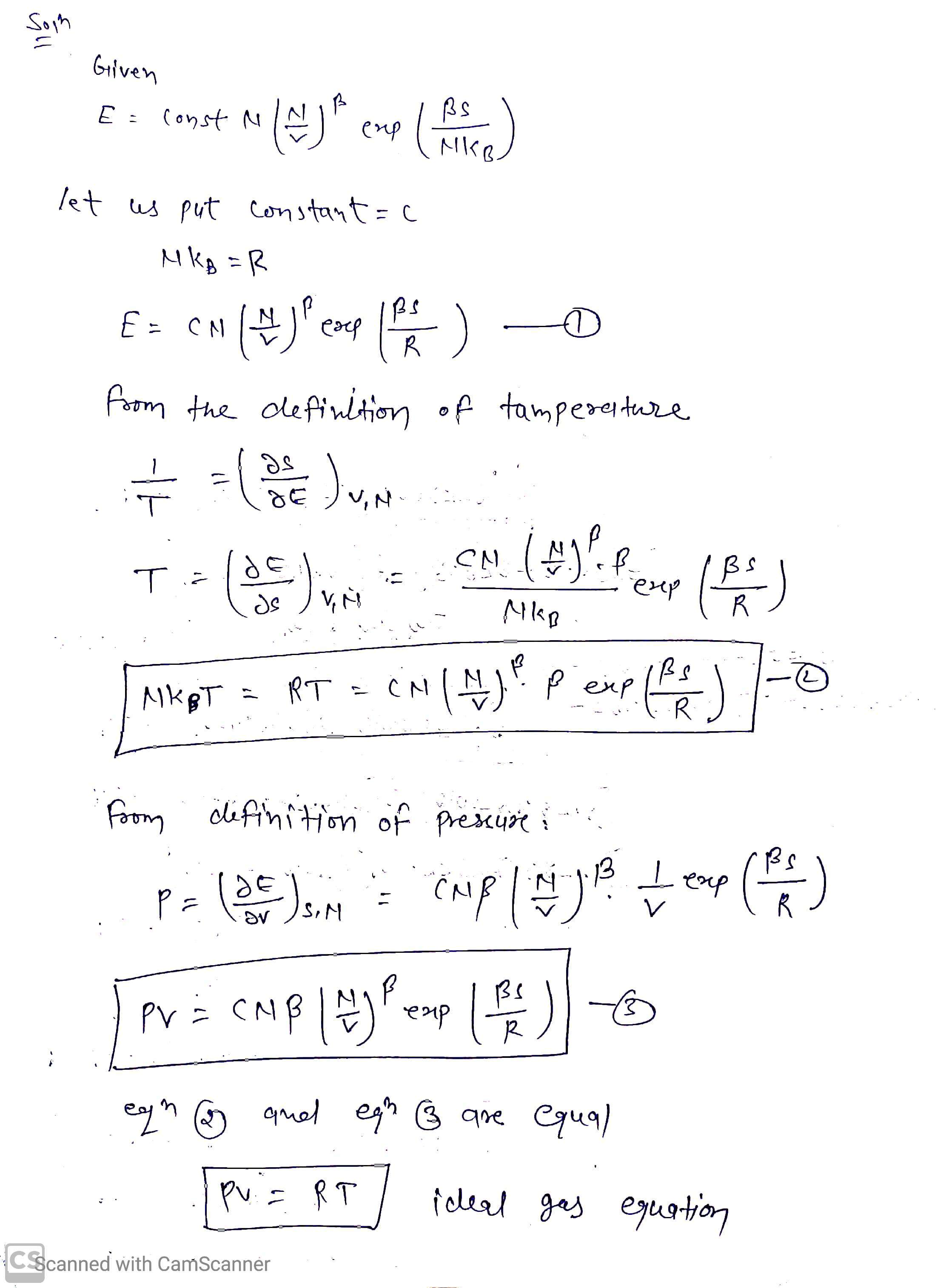
Transcribed Image Text:• 1.11 Derivation of Equation of State
In a certain system the internal energy E is related to the entropy S,
particle number N, and volume V through
BS
exp
E
const N
NkB
(a) Show that the system satisfies the ideal gas law regardless of the
value of the constant B.
(b) Find the coefficient y in the adiabatic equation of state PVY :
const and the molar specific heats Cp and Cv of the system.
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A monatomic ideal gas with volume 0.150 L is rapidly compressed, so the process can be considered adiabatic. If the gas is initially at 1.01 105 Pa and 3.00 102 K and the final temperature is 481 K, find the work done by the gas on the environment, Wenv.Wenv =arrow_forward3. Derive the thermodynamic equation of state for an ideal gas starting from internal energy then express this equation in a form without differentials. = -P +T| T LƏT.arrow_forwardBenzoic acid, C;HsO:H, is typically used as a çalibrant for determining the specific heat of bomb calorimeters. It combusts via the following unbalanced chemical reaction: C,H5O2H (s) + 02(g) → CO2 (g) + H2O(1) 3. For this experimental setup and reaction, the constant volume heat flow is known to be gy = -26.979 kJ. Over the course of the reaction, the temperature rose from 19.863 °C to 22.540 °C. Calculate the heat capacity, Cou, for this calorimeter in kJ °C-!.arrow_forward
- 1. The specific heat capacities (in units of J/g-K) of V, W, and Zn are 0.489, 0.132, and 0.388, respectively. What is the order of the molar heat capacities? Formula weights: V 50.94; W 183.8; Zn 65.4 (c) W>V>Zn (a) V>W>Zn (d) W>Zn>V (b) V>Zn>W (e) Zn>V>Warrow_forwardThe constant heat capacity of perpect with temperature according to the expression a a cample of ZAMOOTMA prers ure fren of punad SOM pound CP / (J K-') gas 2 20.17 0.34 68 CT7K). calculare qi Wi AU and AH when temperature iš raised prom to 100 °C, Ci) at constant ol sig oldenieoh oe 25°0 pressure, (i) dt constant voume.arrow_forward2. For the volumetric properties of pure fluids, two parameters are the volumetric expansivity and the isothermal compressibility, whose formula are given below: 1 av от 1 Ov K=-- Using these parameters, derive a formula for volume as a function of temperature and pressurearrow_forward
- fill in the values for qrxn and delta Hrxn and show how they are calculatedarrow_forwardA quantitity of 100mL of 0.500 MHCl was mixed with 50 mL of 0.500M NaOH in a constant pressure calorimeter of negligible heat capacity. The initial temperature of the HCl and NaOH solutions was the same, 20ºC, and the final temperature of the mixed solution was 22.3ºC. Calculate the heat change for neutralization reaction on a molar baisis.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY