
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
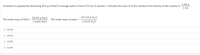
Transcribed Image Text:1.06 g
1 mL
A solution is prepared by dissolving 89.6 g of NaCl in enough water to form 675 mL of solution. Calculate the mass % of the solution if the density of the solution is
58.44 g NacI
18.016 g H20
The molar mass of NaCl
The molar mass of water =
1 mole NaCI
1 mole H20
15.5%
14.6%
13.8%
O 12.5%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.0 chem qarrow_forwardTwo solutions are combined: 611 mL of 139 mM MgSO4 and 566 mL of 174.5 mM NH4OH. How many moles of Mg(OH)2 are formed during the reaction? Report your answer in scientific notation (1.23e4). Remember to identify the limiting reactant.arrow_forwardWhat mass of NaOH would you need to prepare 100.0 mL a 0.50 M solution of NaOH from scratch?arrow_forward
- What is the mass percent of ethanol (CH3CH2OH) if 0.0789 moles of ethanol is added to 0.271 kg of water?arrow_forwardCalculate the Molarity of the solution given the following information. 25g KCI dissolved into 250 mL H20 1 mole KCI = 74.6 g %3Darrow_forwardYou need to make a 0.64 L solution of Mg(NO3)2 that has a nitrate concentration of 0.829 M. What mass of Mg(NO3)2 is required? Report your answer in SI unitsarrow_forward
- 4. Modern pewter is composed of 91.0% Sn, 7.5% Sb and 1.5 % Cu. What mass of pewter contains 150. g of Sb? What mass of Cu is in 212 g of pewter? 91.0g Snx Imole In X I mole In = 108 mole In 118.71g Snarrow_forwardImagine you are preparing a solution of NaCl and a solution of CaCl2. After you prepare them you go out to lunch with your colleagues. When you come back you cannot remember which solution is in which container because you forgot to label the containers before going to lunch. What experiments can you perform to identify each solution?arrow_forwardHow many chloride ions are present in 65.5 mL of 0.210 M AICI3 solution? 4.02 x 1023 chloride ions 5.79 x 1024 chloride ions 2.48 x 1022 chloride ions 8.28 x 1021 chloride ions 1.21 x 1022 chloride ionsarrow_forward
- What is the mass percent of ethanol (CH3CH₂OH) if 0.0750 moles of ethanol is added to 0.350 kg of water?arrow_forwardConsider a solution prepared by combining 45.0 g of BaCl2 with enough water to prepare a 750.0 mL of solution. Assume that the volume of water added was 748 mL. Density of water is 1.00 g/mL. 1) What volume of this solution would be required to prepare 500.0 mL of a solution that is 0.0034 M in BaCl2?arrow_forwardA solution that has 20.54 g of aluminum nitrate in 1575 mL is ? M in Al(NO3)3. A solution that has 2.76 kg of CuSO4·5 H2O in 1.45 L is ? M in copper (II) sulfate pentahydrate. A solution that has 0.005653 mol of Br2 in 10.00 mL is ? M in bromine.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY