
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
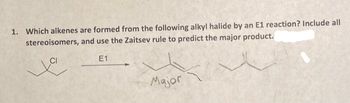
Transcribed Image Text:1. Which alkenes are formed from the following alkyl halide by an E1 reaction? Include all
stereoisomers,
and use the Zaitsev rule to predict the major product.
E1
Major
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- major products, thank you!arrow_forwardThe following compound reacts with NaOMe in an SN2 reaction. Draw the transition state and the final product of the reaction and use the transition state geometry to explain the stereochemical outcome of the reaction. What is the side-reaction that might occur and what would be the major product of that reaction? Br NaOMe ČH3arrow_forwardPlease provide a curved-arrow mechanism for the following reaction. Be sure to use your mechanism and a few words to explain the regiochemistry of the bromines in the final product and why each carbocation intermediate in your mechanism is the preferred intermediate for that step. Include ALL lone pairs and formal charges. Thank you.arrow_forward
- 5.arrow_forwardWhich alkyl bromide(s) can form the alkene under E2 elimination conditions. Na CH,Br CH;CH,OH Br Br, Br в A C These molecules are unreactive B.arrow_forward8. Below are a variety of substitution and elimination reactions. Provide the products for the reactions shown below and identify the mechanism (SN1, SN2, E1, E2). If the products are a mixture of stereoisomers provide both stereoisomers and label their relationship. a) b) d) Br H₂O Br CH3 H₂O deprotonation OCH3arrow_forward
- Predict the major product(s) and provide a mechanism for the reaction below. Includestereochemistry.arrow_forwardA chiral ether of molecular formula C5H10O reacts with hot HI to give a product of molecular formula C5H10I2. Treatment of this compound with hot potassium tert-butoxide produces 1,3-pentadiene. What is the structure of the original ether?arrow_forwardDraw the major product(s) from addition of HBr to the alkene below.arrow_forward
- 7arrow_forwardGive the major product of the following reaction. Br, (1 equiv.) ? hv -Br Br Br- Br There is no reaction under these conditions or the product is not listed here. Br Br Br Br Brarrow_forwardStrong bases are good nucleophiles. Which reaction/s can occur on tertiary alkyl halides when a strong base like KOH is a reagent? A SN1 B) SN2 E1 D E2 E combination of SN & E reactionsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY