
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Analyze and interpret the phase diagram of water and carbon dioxide. Briefly, elaborate your answer to each of the following questions:
1. What is the phase of water at 1 atmospheric pressure at 0oC?
2. What is the phase of water at 1 atmospheric pressure at 100oC?
3. What is the phase of water at 218 atmospheric pressure at 374oC?
4.-5. At what pressure and temperature will water coexist in three distinct phases of matter?
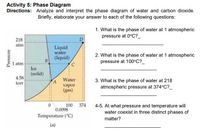
Transcribed Image Text:Activity 5: Phase Diagram
Directions: Analyze and interpret the phase diagram of water and carbon dioxide.
Briefly, elaborate your answer to each of the following questions:
1. What is the phase of water at 1 atmospheric
pressure at 0°C?
218
D
atm
Liquid
water
2. What is the phase of water at 1 atmospheric
(liquid)
B
pressure at 100°C?_
1 atm
Ice
(solid)
4.58
torr
Water
3. What is the phase of water at 218
atmospheric pressure at 374°C?_
vapor
(gas)
100 374
0.0098
4-5. At what pressure and temperature will
water coexist in three distinct phases of
Temperature ("C)
matter?
(a)
Pressure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 17arrow_forwardkJ The enthalpy of vaporization of Substance X is 13.0 and its normal boiling point is 145. °C. Calculate the vapor mol pressure of X at -64. °C. Round your answer to 2 significant digits. atmarrow_forwardkJ The enthalpy of vaporization of Substance X is 5.00 and its normal boiling point is 27. °C. Calculate the vapor pressure of X at -33. °C. mol Round your answer to 2 significant digits. atm x10arrow_forward
- The enthalpy of vaporization for methanol is 35.2 kJ/mol. Methanol has a vapor pressure of 1 atm at 64.7 oC. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 46.3 oC? Give your answer in atmospheres, to the third decimal point.arrow_forwardNeed answers for the blue parts,thank you!!arrow_forward1. Calculate the amount of heat (in kJ) released when converting 150.2 g of water to ice at -23 degrees Celsius. 2. A closed vessel of volume 10.0 L initially contains 1.303 g of water and no water vapor. Please calculate the mass of liquid water remaining once the system reaches equilibrium at 30 degrees Celsius. The vapor pressure of water at that temperature is equal to 0.0418 atm. 3. A person drinks four glasses of cold water (5 degrees C) every day. The volume of each glass is 195. mL. How much heat (in kJ) does the body have to supply to raise the temperature of the water to 37 degrees C (body temperature)? 4.From these data: N2H4(l) + O2(g) à 2N2(g) + 2H2O(l) H= -622.2 kJ/mol H2(g) + ½O2 à H2O(l) H= -285.8 kJ/mol H2(g) + O2(g) à H2O2(l) H= -115 kJ/mol Find H for the reaction: N2H4(l) + 2H2O2(l) à 2N2(g) + 4H2O(l) 5. Consider the following chemical equation. CH4(g) + 2O2(g) à CO2(g) + 2H2O(l) Using the values in the table, calculate…arrow_forward
- 1. Freon-12 (CCl2F2) is banned as a refrigerant because it is harmful to the ozone. a. Sketch the phase diagram of CCl2F2 using the physical constants below Normal boiling point = -29.8 °C Normal melting point = -158 °C Critical temperature = 112 °C Critical pressure = 40.8 atm Triple point (2.42 ×10-6 atm, -157 °C) b. Calculate the heat required to bring 10.0g CCl2F2 from -29.8 °C as a liquid to the gas phase at 10.0 °C. (molar mass 120.91 g/mol) Cs (gas) = 0.074 kJ/mol·K AHvap = 20.1 kJ/mol AHfus = 4.14 kJ/molarrow_forwardHow much energy would need to be added to 930.g of ice at 0.0°C to turn it into liquid water at 0.0°C ? 930. J O 3.89x103 J O 1.86x103 J O 2.10x106 J 3.12x105 Jarrow_forward3. Explain the process of sublimation Name one substance that sublimes at room temperature and pressure.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY