
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Pp1
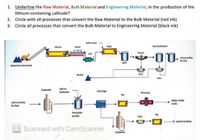
Transcribed Image Text:1. Underline the Raw Material, Bulk Material and Engineering Material, in the production of the
lithium-containing cathode?
2. Circle with all processes that convert the Raw Material to the Bulk Material (red ink)
3. Circle all processes that convert the Bulk Material to Engineering Material (black ink)
Sulfuric Acid
Calcine
Cooler
Roest
Impurity Removal
Thicken
Mil
Lthum Sulfete
Purfed
Leach
Spodumene Concentrate
Residue
Filter
Fiter
um
Evaporate
Carbonate
Centrifuge
Micronize
Battery Grade
uthium Sulfate
Purifed
Precipitate
Dry
Filter
+ Sodium Sulphate
CS Scanned with CamScanner
Crystallse
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- QUESTION 3 A blend of four monodisperse polystyrene (PS) samples was produced. The molecular weights and weight fractions of each monodisperse PS sample are shown in the following table. Sample MW Number (g/mol) fraction 1 10,000 Weight fraction 0.15 MN MW PDI 2 30,000 0.25 3 60,000 0.40 4 100,000 0.20 Blend What is the number-average molecular weight of the blend? 50,000 g/mol ○ 31,000 g/mol ○ 53,000 g/mol ○ 75,000 g/molarrow_forwardI need help with molarity of NaOHarrow_forward111. Food is milled from 6.5 mm to 0.0013 mm using a 10 hp motor. Would this motor be adequate to reduce the size of the particles to 0.00085 mm? Assume Rittinger's equation and that I hp equals to 745.7 W. (Makanan digiling dari 65 mm hingga 0.0013 mm menggunakan motor 10 hp Adakah motor ini mencukupi untuk mengurangkan ukuran zarah hingga 0,00085 mm? Anggap persamaan Rininger dan 1 hp sama dengan 745.7 Warrow_forward
- Consider an methanol(1)/hexane(2) system. a. Calculate (show your work) the saturation pressure [mmHg] of hexane at 72.03635°C b. Calculate T [°C] and y, when P=651.792 mmHg and x=0.25 c. Calculate T [°C] and x1 when P=805.508 mmHg and y=0.450 d. Calculate P [mmHg] and yı when T=66°C and x-0.67465 e. Calculate P [mmHg] and x1 when T=61°C and y=0.13355arrow_forwardThe specific gravity of acetic acid is 1.049. What is the density in Ibm/fts? wwarrow_forwardMaterials Science and Chemical Engineeringarrow_forward
- A metal has density of 19.58 g/cm3, fcc structure and atomic radius of 0.15 nm. Calculate the approximate atomic weight? This question has only one correct answer. Question 10Answer a. atomic weight =253.3 b. atomic weight =225.2 c. atomic weight =197.0 d. atomic weight =112.6 % e. atomic weight =323.7arrow_forwardCite four reasons landfills remain a viable hazardous waste management alternative.arrow_forwardWhat is the importance of the catalyst in the biodiesel production process? O Making soap All of these O Increase the acidity of the reaction O Accelerate the reactionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The