Question
Can you help me solve this question clearly with steps on how to do it, you can take write the first part as ψ2,0,0= C(1-br)e^-br where b= Z/2ao to make it easier to solve
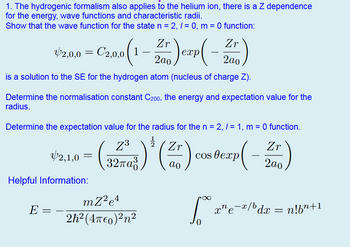
Transcribed Image Text:1. The hydrogenic formalism also applies to the helium ion, there is a Z dependence
for the energy, wave functions and characteristic radii.
Show that the wave function for the state n = 2, 1 = 0, m = 0 function:
Zr
$2.00- Czao (1-2)crp (-Z)
42,0,0 = 2,0,0
2ao
exp
is a solution to the SE for the hydrogen atom (nucleus of charge Z).
Determine the normalisation constant C200, the energy and expectation value for the
radius.
Determine the expectation value for the radius for the n = 2, /= 1, m = 0 function.
Zr
Z3
Zr
2,1,0
=
cos exp
32πα
ao
P(-
2ao
Helpful Information:
E
_
mZ2e4
2h² (4π0)²n²
∞
xne¯x/bdx = n!b”+1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps
