
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
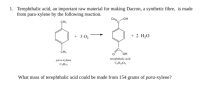
Transcribed Image Text:1. Terephthalic acid, an important raw material for making Dacron, a synthetic fibre, is made
from para-xylene by the following reaction.
он
+ 30,
+ 2 H,0
CH3
OH
para-sylene
terephthalic acid
Cll
What mass of terephthalic acid could be made from 154 grams of para-xylene?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Acrylonitrile is an important building block for synthetic fibers and plastics. Over 2 billion pounds of acrylonitrile are produced in the United States each year. The compound is synthesized from propene in the following reaction:2C3H6 + 2NH3 + 3O2 → 2C3H3N + 6H2OWhat mass of acrylonitrile can be prepared from 150.0 kg of propene, 600.0 kg of ammonia, and 100.0 kg of oxygen?arrow_forwardHeptacarbon octahydride is an effective gasoline additive. In the presence of oxygen gas (and a spark), it undergoes complete combustion.(b) Calculate the theoretical yield of water (in g) when 500.0 g of heptacarbon octahydride reacts with excess O2.arrow_forwardAluminum chloride is made from scrap aluminum through the following balanced reaction. 2 Al (s) + 3 Cl2 (g) --> 2 AlCl3 (s) a. If you begin with 19.7 moles of aluminum and 9.88 grams of chlorine, how much aluminum trichloride is produced? b. What is the mass of excess reagent?arrow_forward
- Q4. Some airbags in cars contain sodium azide (NaN3). (a) Sodium azide is made by reacting dinitrogen monoxide gas with sodium amide (NANH2) as shown by the equation. 2NANH2 + N20 → NaN3 + NAOH NH3 Calculate the mass of sodium amide needed to obtain 550 g of sodium azide, assuming there is a 95.0% yield of sodium azide. Page 5 of 12 Give your answer to 3 significant figures. (b) If a car is involved in a serious collision, the sodium azide decomposes to form sodium and nitrogen as shown in the equation. 2NAN3(s) 2Na(s) + 3N2(g) The nitrogen produced then inflates the airbag to a volume of 7.50 x 10-2 m3 at a pressure of 150 kPa and temperature of 35 °C. Calculate the minimum mass of sodium azide that must decompose. (The gas constant R = 8.31 J K-1 mol-1) (Hint: find out the number of moles of Nzusing Ideal gas equation, and determine the number of moles and mass of NaN3 from the balanced equation)arrow_forwardCombining 0.266 mol Fe,O, with excess carbon produced 20.0 g Fe. Fe, O, + 3 C → 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? theoretical yield: molarrow_forwardWhat mass of H2O is produced from 100 g of H2 in the foillowing reaction. 2H2 + O2 → 2H2Oarrow_forward
- 1. Urea can be prepared by reacting ammonia with carbon dioxide. 2 NH3 (g) + CO2 (g) ® (NH2)2C=O (aq) + H2O (l) Calculate the mass of urea (in grams) which is formed from the reaction of 375 g of ammonia with 650 g of CO2.arrow_forward2 Al (s) + 3 Br2 -----------> 2AlBr3 (s) How many grams of aluminum bromide are produced by the complete reaction of 0.557 grams of aluminum?arrow_forwardHow many grams of CaCO3 will be produced if we produce 62.06 grams of NaCl in the reactionCaCl2 + Na2CO3 ———> 2 NaCl + CaCO3arrow_forward
- 5. An organic compound containing only C, H, and possibly O was subjected to combustion analysis. A sample weighing 0.7585 g yielded 1.295 g CO₂ and 0.464 g H₂O. What is the empirical formula of the compound?arrow_forwardABS plastic is a polymer consisting of three monomer units: acrylonitrile (C₃H₃N), butadiene (C₄H₆), and styrene (C₈H₈). A sample of ABS plastic contains 13.7% N by mass. It took 1.56 g of Br₂ to react completely with a 2.00 g sample of ABS plastic. Bromine reacts 1:1 (by moles) with the butadiene units in the polymer and nothing else, so bromination is a method for determining the quantity of butadiene in the polymer. What is the percent by mass of styrene in the polymer?arrow_forwardBalance and complete the following combustion reaction. Give the sum (one number only) t e t r a c a r b o n h e x a h y d r i d e ( g ) + o x y g e n ( g ) ⟶ ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY