
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
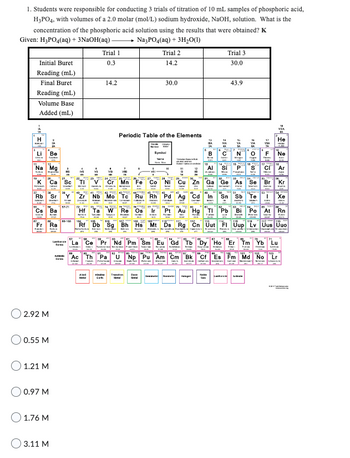
Transcribed Image Text:1. Students were responsible for conducting 3 trials of titration of 10 mL samples of phosphoric acid,
H3PO4, with volumes of a 2.0 molar (mol/L) sodium hydroxide, NaOH, solution. What is the
concentration of the phosphoric acid solution using the results that were obtained? K
Given: H₂PO4(aq) + 3NaOH(aq) -
Na3PO4(aq) + 3H₂O(1)
Initial Buret
Reading (mL)
Final Buret
Reading (mL)
Volume Base
Added (mL)
2 =>
Na Mg
Rb Sr
2.92 M
Cs Ba
0.55 M
1.21 M
Be
0.97 M
1.76 M
Ca Sc
3.11 M
Ra
La
91
AM
Hf
Ac
Trial 1
0.3
14.2
TI
Zr Nb Mo Tc
[81
$4
Cr Mn Fe
Re Os
Rf Db Sg Bh Hs
Periodic Table of the Elements
T
Trial 2
14.2
A
Basic
D
30.0
Symbol
10
77 78
224,
Co Ni Cu Zn Ga
B
14
Neble
Gas
Trial 3
30.0
43.9
Ru Rh Pd Ag Cd In Sn Sb Te
M
с N
Expe
201
VIA
Sm
Nd Pm
Eu Gd
Dy Ho
"Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
15
VIA
M
He
Ge As Se Br Kr
Xe
Pt Au Hg
Ds Rg Cn Uut FI Uup Lv Uus Uuo
Ne
"Er Tm Yb
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of acetic acid is titrated with a standardized NAOH solution. Before the end-point of the titration, which of the following must be true? HC,H3O, (aq) + NAOH (aq) → NaC,H3O2 (aq) + H2O (1) Note: [X] = molar concenctration of X %3D O [H+] > [OH-] [-HO] > [+H] O %3D [-HO] = [+H] Oarrow_forwardIf 100.0 mL of 0.250 M HBr is titrated with 0.150 M KOH answer the following questions. What is the pH of the solution before the titration begins? How many milliliters of NaOH must be added to reach the equivalence point? HBr (aq) + KOH (aq) KBr (aq) + H2O (l) What is the pH of the solution at the equivalence point? What is the pH of the solution when 30.0 mL of KOH has been added? What is the pH of the solution when 180.0 mL of KOH has been added?arrow_forwardA 2.00 L solution contains 0.545 M NH3 and 0.641 M NH4A. (The Kb of NH3 is 1.86⋅10−51.86⋅10-5). What is the pH of this solution? What is the pH of this solution after 0.121 mol of HCl are added to the solution? What is the pH of this solution after 0.242 mol of HCl are added to the solution?arrow_forward
- 2. If you make a solution by mixing 25.mL of 0.50 M acetic acid (CH3COOH) and 25 mL of 0.30 M sodium acetate (CH3COONa) together in a beaker: a. The Ka of acetic acid is 1.8 x 105. What is the pH of the solution that you made? What is the degree of ionization? CH&COOH+ CHeCOONat lOH 4 g CH300Harrow_forward11arrow_forwardA student peforms a titration, titrating 25.00 mL of a weak monoprotic acid, HA, with a 1.14 M solution of NaOH. They collect data, plot a titration curve and determine the values given in the below table. During the titration the following reaction occurs HA + NaOH ⇒ NaA + H2OWhat is the concentration of A- (the conjugate base of the weak acid) at the equivalence point? ml NaOH added pH Hal-way point 18.71 3.41 Equivalence point 37.41 8.53arrow_forward
- Can you help me answer the PH=arrow_forward100.0 mL of HCI required 25.00 mL of 0.2000 M Mg(OH)2 to reach pH 7. What is the concentration of the HCI ? 2 HCI + Mg(OH)₂ MgCl₂ + 2 H₂O The neutralization equation is There is enough information to calculate the moles of Mg(OH)2 ( L Mg(OH)₂ (- concentration = Then use stoichiometry to convert moles of Mg(OH)₂ into moles of HCI mol HCI a. acid i. 0.2000 p. 0.01000 mol Mg(OH)₂ (- b. base c. HCI d. NaOH mol Mg(OH)2 We now can calculate the concentration of HCI since we have both the moles of HCI and the volume of HCI j. 1.000 k. 0.005000 q. 0.001000 1 L mol HCI LHCI r. 58.31 = mol Mg(OH)2 e. NaCl I. 100.0 f. H₂O m. 0.1000 s. 5.831 = g. 25.00 mol/L n. 0.05000 h. 0.02500 mol Mg(OH)2 o. 2.000 mol HCIarrow_forwardAn analytical chemist is titrating 143.6 mL of a 0.7000M solution of piperidine (C,H10NH) with a 0.4000M solution of HNO3. The p K, of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 267.7 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places. pHarrow_forward
- ssume you dissolve 0.235 g of the weak acid benzoic acid, C6H5CO2H, in enough water to make 7.00 ✕ 102 mL of solution and then titrate the solution with 0.153 M NaOH. C6H5CO2H(aq) + OH-(aq) C6H5CO2-(aq) + H2O(ℓ) What are the concentrations of the following ions at the equivalence point? Na+, H3O+, OH-C6H5CO2- M Na+ M H3O+ M OH- M C6H5CO2-arrow_forward20. mL of 0.250 M NH3 is titrated with 0.500 M of HCl solution, what is the pH of the resulting solution when 0mL, 5.0 mL, 10.0 mL and 12 mL of HCl was added into the NH3solution? KB(NH3) = 1.8 x 10-5arrow_forwardAssume you dissolve 0.235 g of the weak acid benzoic acid, C6H5CO2H, in enough water to make 3.00 ✕ 102 mL of solution and then titrate the solution with 0.148 M NaOH. C6H5CO2H(aq) + OH-(aq) C6H5CO2-(aq) + H2O(ℓ) What are the concentrations of the following ions at the equivalence point? Na+, H3O+, OH-C6H5CO2- M H3O+ M OH-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY