
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
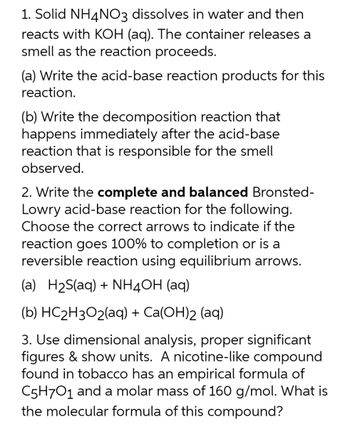
Transcribed Image Text:1. Solid NH4NO3 dissolves in water and then
reacts with KOH (aq). The container releases a
smell as the reaction proceeds.
(a) Write the acid-base reaction products for this
reaction.
(b) Write the decomposition reaction that
happens immediately after the acid-base
reaction that is responsible for the smell
observed.
2. Write the complete and balanced Bronsted-
Lowry acid-base reaction for the following.
Choose the correct arrows to indicate if the
reaction goes 100% to completion or is a
reversible reaction using equilibrium arrows.
(a) H₂S(aq) + NH4OH(aq)
(b) HC₂H3O2(aq) + Ca(OH)2 (aq)
3. Use dimensional analysis, proper significant
figures & show units. A nicotine-like compound
found in tobacco has an empirical formula of
C5H7O1 and a molar mass of 160 g/mol. What is
the molecular formula of this compound?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the difference between a strong acid such as hydrochloric acid, HCl(aq), and a weak acid such as acetic acid, HC2H3O2(aq)?arrow_forwardHow many millilitres of 0.450 mol L−1 H2SO4(aq) are required to completely neutralize 46.7 mL of 0.930 mol L−1 KOH(aq) ? Hint: Write a balanced chemical equation for the reaction. Give your answer in mL, accurate to three significant figures. Do not include units as part of your answer.arrow_forwardFor each of the following reactions, suggest two soluble ionic compounds that, when mixed together in water, result in the net ionic equation given: (a) 2 Ag+ (aq) + CO3²¯ (aq) → Ag₂CO3(s) (b) Mg²+ (aq) + 2 OH¯(aq) → Mg(OH)₂(s), the suspension present in milk of magnesia 3+ (c) 3 Ca³+ (aq) + 2 PO2 (aq) → Ca3(PO4)2(s), gypsum, a component of concretearrow_forward
- A 0.124 M NaOH(aq) solution was used to titrate 20.00 mL of an acetic acid (CH3COOH) solution that has an unknown concentration. The equivalence point is reached after adding 15.34 mL of NaOH(aq). a) Write out the complete balanced equation for the reaction that occurs in this titration. b) How man moles of NaOH were added to the acetic acid solution? c) How many moles of acetic acid were in the original 20.00 mL solution? d) What was the concentration of acetic acid in the orignial 20.00 mL solution?arrow_forwardAs shown in the following Figure, an aqueous solution of Nal is poured into an aqueous solution of Pb(NO3)2 which results in the formation of a yellow precipitate. Assuming stoichiometric amounts of Nal and Pb(NO3)2 are reacted, give the chemical formula of the yellow precipitate and the chemical formula of all molecules and/or ions that are present in the solution (You may use the solubility matrix to answer this question). (b)arrow_forwardWhen sodium acetate, CH3COONa(s), is added to CH3COOH (aq), why does the pH of the solution increase?arrow_forward
- (a) The sodium ion did not take part in this chemical reaction. What do we call such an ion? (b) Draw a simple diagram which shows how the sodium ion mixes with water in solution. What do we call this physical process?arrow_forwardMuch of the sulfur used in the United States comes from the hydrogen sulfide contaminant that makes "sour" natural gas smell bad. Hydrogen sulfide is separated from the other components of natural gas mostly by taking advantage of its acid-base reaction with aqueous ethanolamine: HO(CH,),NH,(aq)+H,S(g) → HO(CH,),NH,(aq)+HS (aq) Suppose an engineer decides to study the rate of this reaction. She prepares four reaction vessels with 167.6 mL of ethanolamine solution and 20.9 g of hydrogen sulfide gas each. The volume and temperature of each vessel is shown in the table below. Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. vessel volume temperature A B C D 5.0 L 5.0 L 5.0 L 5.0 L 49, °C 48. °C 51. °C 50. °C X initial rate of…arrow_forwardBy titration, it is found that 39.1 mL of 0.161 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCl solution.arrow_forward
- Give the answers that should be filled in the blanks below: Acetic acid solution: pH = 5.80; (a) [H+] = M; (b) [OH-] = M Ammonia solution: [H+] = 5.90 x 10-9 M; (c) pH = ; (d) pOH =arrow_forwardHow many millilitres of 0.590 mol L−1 H2SO4(aq) are required to completely neutralize 28.1 mL of 0.570 mol L−1 KOH(aq) ? Hint: Write a balanced chemical equation for the reaction. Give your answer in mL, accurate to three significant figuresarrow_forwardThe following chemical reaction takes place in aqueous solution: CuBr2(aq)+2KOH(aq)→CuOH2(s)+2KBr(aq).Write the net ionic equation for this reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY