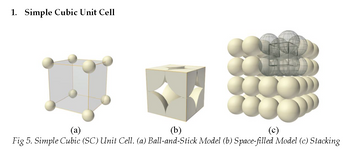
e. Consider one unit cell and assume the length of the side of the cube is “a”. Remember that “a” is the distance between the centers of two adjacent atoms. How long is “a”, the edge of a unit cell, in terms of radius, r, of an atom? Write also your answer in the summary table.
Answer: __________
f. Based on the earlier questions, a simple cubic cell has the equivalent of only 1 atom. Recall the volume of sphere with radius, r, is expressed as V = 4/3 πr3. With this information, find the total volume of all the spheres in this unit cell, expressed in terms of r. (Hint: To do this, take the total number of atoms and multiply it by the volume of one atom, with radius, r)
Answer: __________
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

- Please help. This problem involves finding the radius and volume of a platinum atom using its density. Thank you.arrow_forwardFor this problem, we want to estimate the answer, so our assumptions may be a little unrealistic. Suppose we want to estimate how much air we need to send to a space station, assuming we cannot recycle air. Suppose four astronauts are in a spherical space station. If each of them typically breathes about 500 cm³of air with each breathe, and take 15 breathes per minute (average resting value): a. What is the volume of air you would need in the space station if these four astronauts stayed for a full year? b. If the density of air is 1.25 kg/m³ and it costs (thanks to SpaceX) a mere $100/kg to send objects to the space station, how much money would the astronaut air supply cost?arrow_forwardFor the remaining questions, you need to use Figure 1.4 to obtain the necessary orders of magnitude of lengths, masses, and times.About how many floating-point operations can a supercomputer perform each year?arrow_forward
- a. 2πR2+2πRh2πR2+2πRh b. 2πR2+2πh2πR2+2πh c. πR2hπR2h d. πD2+πDhπD2+πDh e. 2πR+2πRharrow_forwardNeeds Complete typed solution with 100 % accuracy.arrow_forwardA cylinder containing 239.6 cubic centimeter of gas at a pressure of 342 kPa when its temperature is 406 K. Given that its volume is unchanged when the pressure was increased by a factor of 1.2, Determine the new Temperature of the gas (In Kelvin). Note: Your answer must be in Kelvin, however, do not include the unit, just enter the magnitude that corresponds to the final volume in Kelvin. Round your answer to 2 decimal points Round your answer to 2 decimal pointsarrow_forward
- A. On the average, what volume of blood, in liters, does the heart pump during each beat? B. On the average, what volume of blood, in cubic centimeters, does the heart pump during each beat?arrow_forwardSmall animals eat much more food per kilogram of body mass than do larger animals. The basal metabolic rate (BMR) is the minimal energy intake necessary to sustain life in a state of complete inactivity. The table lists the BMR in kilocalories per day, the mass, and the surface area for 4 animals. What is the BMR per square meter of the surface area for the mouse?arrow_forwardA) Given the vectors A⃗ A→ and B⃗ B→ shown in the figure ((Figure 1)), determine the magnitude of B⃗ −A⃗ B→−A→. B) Determine the direction of B⃗ −A⃗ C) Determine A⃗ −B⃗ A→−B→ without using your answer in parts AA and BB. Then compare your results and see if they are opposite. Determine the magnitude of A⃗ −B⃗ A→−B→. D) Determine the direction of A⃗ −B⃗ A→−B→.arrow_forward
- The formula for differential volume in a spherical coordinate. a. ? sin ? ?? ?? ??b. ? sin ? ?? ?? ??c. ? sin ? ?? ?? ??d. ? sin ? ?? ?? ??arrow_forwardThe Statue of Liberty in New York City is approximately 305 ft305 ft tall. How many U.S. dimes would be in a stack of the same height? Each dime is 1.35 mm1.35 mm thick. number of dimes: Each dime has a mass of 2.268 g.2.268 g. How much would the stack of dimes from the previous question weigh? mass: g What is the value, in dollars, of the same stack of dimes? value: dollars The 2017 U.S. gross domestic product (GDP) was valued at 19,390,604,000 dollars.19,390,604,000 dollars. How many Statue of Liberty‑height stacks of dimes are needed to match the GDP in value? number of stacks:arrow_forwardI need help with knowing what to cirlce. The " increases decreases" part.arrow_forward