
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
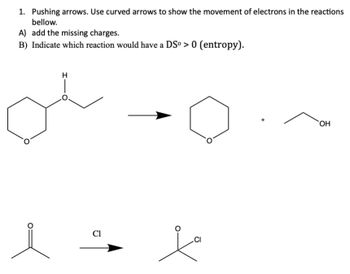
Transcribed Image Text:1. Pushing arrows. Use curved arrows to show the movement of electrons in the reactions
bellow.
A) add the missing charges.
B) Indicate which reaction would have a DSº > 0 (entropy).
i
Cl
CI
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- acid. b- 6. Consider the reaction below: (a). Highlight (or circle) the bonds that are broken/changed. (b). Identify the reaction type. (c). Explain why this makes Vitamin Ca good antioxidant. HO но Но—с-н Но-с-н ČH,OH ČH;OH !!! I 8 | 6 | OLarrow_forwardA reaction is endothermic. If we add heat to the reaction, will it shift to the right (towards products) or to the left (towards reactants)? ?+ℎ???↔?+?arrow_forwardA reaction has a rH value of -32.5 kJ mol-1. Is this reaction endothermic or exothermic?arrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!arrow_forwardFill in the missing information for each chemical reaction by adding the skeletal structures and IUPAC names for the reactants and/or products missing (indicated by a “?” for a missing skeletal structure or ____ for a missing name) needed to complete the reaction. Additionally, name the type of reaction when asked for.arrow_forwardGive the missing reactant: .CO,CH, OA. I OB. II O C. III OD. IV + [ ³ ]- ? || heat = A CO₂CH3 IVarrow_forward
- When a mixture of hydrogen and bromine is maintained at normal atmospheric pressure and heated above 200. °C in a closed container, the hydrogen and bromine react to form hydrogen bromide and a gas-phase equilibrium is established. Write a balanced chemical equation for the equilibrium reaction. Use bond enthalpies from Table 6.2 ( Sec. 6-6b) to estimate the enthalpy change for the reaction. Based on your answers to parts (a) and (b), which is more important in determining the position of this equilibrium, the entropy effect or the energy effect? In which direction will the equilibrium shift as the temperature increases above 200. °C? Explain. Suppose that the pressure were increased to triple its initial value. In which direction would the equilibrium shift? Why is the equilibrium not established at room temperature?arrow_forwardFor which of the following reactions is AHn equal to AH; of the product(s)?You do not need to look up any values to answer this question. Check all that apply. > View Available Hint(s) O Na(s) + F2 (1)→NAF(s) O Na(s) + F2 (g)→NAF(s) C(s, graphite) + 0,(g) →CO,(g) 2Na(s) + F2 (g)→2NAF(s) CACO3 (g)→Ca0 + CO2 (g) O CO(g) + O2(g)→CO2(g)arrow_forwardQ6. Complete the sentences from the reading above with your answers from Q5 (Q5. The following fictitious unbalanced Redox reaction is made using some of the 1001GRC teachers’ initials and oxygen. Show ALL steps that were involved in balancing this equation: ChO3- + Sh → Ch- + Sh2+ (acidic solution) ) Choose from the following: reduced, oxidised, reducing agent, oxidising agent. The Ch is to Ch- and acts as a(n) . Sh is to Sh2+ and acts as a(n) (4).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning