
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I am trying to figure out the movement of the electrons and how the the reactant gets oxydized.
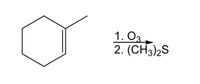
Transcribed Image Text:1. O3
2. (CH3)2S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the concentration of 10.00 mL of HBr if it takes 5.00 mL of a 0.253 M LiOH solution to neutralize it? a) Determine the moles of base: b) Determine the mole ratio of acid base: c) Determine the concentration of the acid:arrow_forwardGoing from an ionic equation to a net ionic equation involves cancelling out the........ titration Molarity oxidation reduction precipitate spectator ionsarrow_forwardWhat is the molecular and net ionic equation for calcium ions in urine react with oxalic acid (H2C2O4) to form solid kidney stones in the body (precipitate)?arrow_forward
- How do electrons work in total ionic equations?arrow_forwardPlease explain what a redox reaction is and how would you recognise it?arrow_forward1. Which is statement is INCORRECT? a. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. b. Reduction is a process in which an atom gains an electron and therefore decreases (or reduces its oxidation number). c. Oxidation is a process in which an atom gains an electron and therefore increases its oxidation number. d. An electron-donating species which tends to hand over electrons can be referred to as a reducing agent. 2. What chemical process must always accompany a reduction process? a. oxidation c. reduction b. balancing reaction d. half-reactionarrow_forward
- 36) In water a completely breaks into ions in solution. weak electrolyte nonconductor suspension strong electrolyte nonelectrolytearrow_forwardWhich of the following metals can displace nickel from an aqueoussolution but not manganese? a. chromiumb. sodiumc. goldd. cobalte. aluminumf. copperg. barium Plz give me all possible answersarrow_forwardI need help with the following question, please! I was sick for a couple days so I don't understand how to do this question at allarrow_forward
- Indicate whether the statement below is true or false. The sum of the oxidation numbers in polyatomic ions must be equal to the charge on the ion. True Falsearrow_forwardthe anwer givin was incorrect. oxidation was correct and strong weak mixture was incorrect.arrow_forwardBased on your answer in the previous question, is the reaction between sulfuric acid and sodium bicarbonate a redox reaction? O No ch of the following statements is/are TRUE regarding bases? Vapose all that applies.) Yesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY