
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Solve 1.
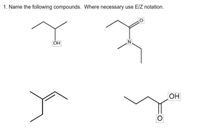
Transcribed Image Text:1. Name the following compounds. Where necessary use E/Z notation.
ÓH
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Convert 38\ deg Convert K.arrow_forwardThe density of a gas is 1.76 g/L at a particular pressure and 10.0°C. What is its density in g/L at 1.5 times this pressure and 20°C. Express your answer in decimal notation rounded to three significant figures.arrow_forwardPlease help me find volume 285m^3 in each of these unit's with work! Thanks so mucharrow_forward
- A cylinder measuring 9.8 cm wide and 12. cm high is filled with gas. The piston is pushed down with a steady force measured to be 18. N. piston cylinder gas Calculate the pressure of the gas inside the cylinder. Write your answer in units of kilopascals. Round your answer to 2 significant digits. XPa Explanation Check O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy| Accessibility lenovo .uddle WebEx at 3pm (dia' just before 3pm) 1-650-479-3208 Access Code (meeting Number): 737 129 915 Dorsi 3134arrow_forwardA 1.25 L bottle of soda exerts a force of ________ N and a pressure of ________ Pa when placed on a table. The round cylindrical bottle has a diameter of 10.0 cm and a height of 25.0 cm. Assume that the density of the soda is 1.0 g/mL, and that the mass of the plastic bottle is negligible. Remember, the acceleration due to gravity is 9.81 m/s2; 1 Newton = 1 kg m/s2, 1 Pascal = 1 kg/(m s2), A = π r2, V = π r2hHOW DO I SOLVE THIS QUESTION?arrow_forwardPlease show me how to solve this. Thanks.arrow_forward
- Hello, can this problem please be broken down for me. In the cylinder shown the pressure is 58 psi at 409 mL. What would the new pressure be if the cylinder volume was adjusted to 5 mL under constant temperature? Answer unitsarrow_forwardWhat is the answer? 1,844 mL of a gas is at 47.4°C. At what temperature would the volume of the gas increase to 3.74 L?Round your final answer to 2 decimal places.arrow_forwardPlease send me the question in 20 minutes it's very urgent plzarrow_forward
- A 0.28 g sample of carbon dioxide has a volume of 1.04 L and pressure of 1.09 atm. What is the temperature of the gas in K? R = 0.0821 Present the answer with one digit after the decimal point.arrow_forwardA chemical engineer must report the average volume of a certain pollutant produced by the plants under her supervisio Here are the data she has been given by each plant: volume of plant pollutant Lincoln 0.521 L Doheny 3.453 L Allegheny 1.654 L What average volume should the chemical engineer report? Be sure your answer has the correct number of significant digits. x10 ?arrow_forwardConvert 26.31 mg into amu, using dimensional analysis.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY